Root pruning improves maize water-use efficiency by root water absorption
- PMID: 36684736
- PMCID: PMC9845614
- DOI: 10.3389/fpls.2022.1023088
Root pruning improves maize water-use efficiency by root water absorption
Abstract
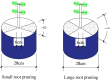
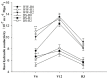
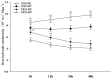
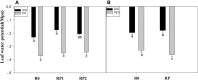
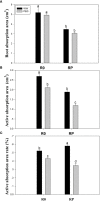
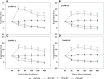
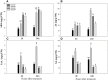
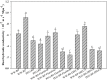
Root systems are an important component of plants that impact crop water-use efficiency (WUE) and yield. This study examined the effects of root pruning on maize yield, WUE, and water uptake under pot and hydroponic conditions. The pot experiment showed that root pruning significantly decreased root/shoot ratio. Both small root pruning (cut off about 1/5 of the root system, RP1) and large root pruning (cut off about 1/3 of the root system, RP2) improved WUE and root hydraulic conductivity (Lpr) in the residual root system. Compared with that in the un-cut control, at the jointing stage, RP1 and RP2 increased Lpr by 43.9% and 31.5% under well-watered conditions and 27.4% and 19.8% under drought stress, respectively. RP1 increased grain yield by 12.9% compared with that in the control under well-watered conditions, whereas both pruning treatments did not exhibit a significant effect on yield under drought stress. The hydroponic experiment demonstrated that root pruning did not reduce leaf water potential but increased residual root hydraulic conductivity by 26.2% at 48 h after root pruning under well-watered conditions. The foregoing responses may be explained by the upregulation of plasma membrane intrinsic protein gene and increases in abscisic acid and jasmonic acid in roots. Increased auxin and salicylic acid contributed to the compensated lateral root growth. In conclusion, root pruning improved WUE in maize by root water uptake.
Keywords: abscisic acid; jasmonic acid; leaf water potential; root hydraulic conductivity; root pruning.
Copyright © 2023 Yan, Zhang, Li, Zhang, Ren, Chen, Cai and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Higher Water Absorption Capacity of Small Root System Improved the Yield and Water Use Efficiency of Maize.Plants (Basel). 2022 Sep 2;11(17):2300. doi: 10.3390/plants11172300. Plants (Basel). 2022. PMID: 36079683 Free PMC article.
-
Seedling-Stage Deficit Irrigation with Nitrogen Application in Three-Year Field Study Provides Guidance for Improving Maize Yield, Water and Nitrogen Use Efficiencies.Plants (Basel). 2022 Nov 7;11(21):3007. doi: 10.3390/plants11213007. Plants (Basel). 2022. PMID: 36365460 Free PMC article.
-
Cultivar replacement increases water use efficiency in foxtail millet in Shaanxi Province, China.Plant Physiol Biochem. 2021 Jul;164:73-81. doi: 10.1016/j.plaphy.2021.04.036. Epub 2021 May 5. Plant Physiol Biochem. 2021. PMID: 33971461
-
[Effect of nitrogen nutrition on endogenous hormone content of maize under soil drought conditions].Ying Yong Sheng Tai Xue Bao. 2003 Sep;14(9):1503-6. Ying Yong Sheng Tai Xue Bao. 2003. PMID: 14733008 Chinese.
-
The role of root size and root efficiency in grain production, and water-and nitrogen-use efficiency in wheat.J Sci Food Agric. 2023 Nov;103(14):7083-7094. doi: 10.1002/jsfa.12794. Epub 2023 Jul 3. J Sci Food Agric. 2023. PMID: 37332073
Cited by
-
The Molecular Mechanism and Effects of Root Pruning Treatment on Blueberry Tree Growth.Plants (Basel). 2025 Jul 23;14(15):2269. doi: 10.3390/plants14152269. Plants (Basel). 2025. PMID: 40805616 Free PMC article.
-
Evaluation of drought-tolerant chickpea genotypes (Cicer arietinum L.) using morphophysiological and phytochemical traits.Front Plant Sci. 2025 Apr 9;16:1529177. doi: 10.3389/fpls.2025.1529177. eCollection 2025. Front Plant Sci. 2025. PMID: 40271442 Free PMC article.
-
A Multi-Omics Analysis Revealed the Diversity of the MYB Transcription Factor Family's Evolution and Drought Resistance Pathways.Life (Basel). 2024 Jan 18;14(1):141. doi: 10.3390/life14010141. Life (Basel). 2024. PMID: 38255756 Free PMC article.
References
-
- Abdelhakam S., Rabei S. H., Nada R. M., Abogadallah G. M. (2021). The complementary role of root and leaf PIP1 and PIP2 aquaporins drives the anisohydric behavior in Helinathus annuus L. Environ. Exp. Bot. 182, 104314. doi: 10.1016/j.envexpbot.2020.104314 - DOI
-
- Adie B. A. T., Perez-Perez J. N., Perez-Perez M. M., Godoy M., Sanchez-Serrano J. J., Schmelz E. A., et al. . (2007). ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in arabidopsis. Plant Cell. 19, 1665–1681. doi: 10.1105/tpc.106.048041 - DOI - PMC - PubMed
-
- Aroca R. (2006). Exogenous catalase and ascorbate modify the effects of abscisic acid (ABA) on root hydraulic properties in Phaseolus vulgaris L. plants. J. Plant Growth Regul. 25, 10–17. doi: 10.1007/s00344-005-0075-1 - DOI
-
- Aroca R., Vernieri P., Irigoyen J. J., Sanchez-Diaz M., Tognoni F., Pardossi A. (2003). Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci. 165, 671–679. doi: 10.1016/s0168-9452(03)00257-7 - DOI
LinkOut - more resources
Full Text Sources

