Multiscale imaging reveals the presence of autophagic vacuoles in developing maize endosperm
- PMID: 36684761
- PMCID: PMC9853038
- DOI: 10.3389/fpls.2022.1082890
Multiscale imaging reveals the presence of autophagic vacuoles in developing maize endosperm
Abstract
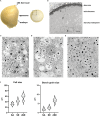
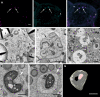
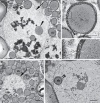
Cereal endosperm is solely devoted to the storage of proteins and starch that will be used by the embryo upon germination. The high degree of specialization of this tissue is reflected in its endomembrane system, in which ER derived protein bodies and protein storage vacuoles (PSVs) are of particular interest. In maize seeds, the main storage proteins are zeins, that form transport incompetent aggregates within the ER lumen and finally build protein bodies that bud from the ER. In contrast to the zeins, the maize globulins are not very abundant and the vacuolar storage compartment of maize endosperm is not fully described. Whereas in other cereals, including wheat and barley, the PSV serves as the main protein storage compartment, only small, globulin-containing PSVs have been identified in maize so far. We present here a multi-scale set of data, ranging from live-cell imaging to more sophisticated 3D electron microscopy techniques (SBF-SEM), that has allowed us to investigate in detail the vacuoles in maize endosperm cells, including a novel, autophagic vacuole that is present in early developmental stages.
Keywords: autophagy; lytic vacuole; maize endosperm; multiscale imaging; storage vacuole.
Copyright © 2023 Arcalís, Hörmann-Dietrich and Stöger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Delivery of prolamins to the protein storage vacuole in maize aleurone cells.Plant Cell. 2011 Feb;23(2):769-84. doi: 10.1105/tpc.110.082156. Epub 2011 Feb 22. Plant Cell. 2011. PMID: 21343414 Free PMC article.
-
RNA targeting to a specific ER sub-domain is required for efficient transport and packaging of α-globulins to the protein storage vacuole in developing rice endosperm.Plant J. 2012 May;70(3):471-9. doi: 10.1111/j.1365-313X.2011.04880.x. Epub 2012 Jan 18. Plant J. 2012. PMID: 22168839
-
Fusion, rupture, and degeneration: the fate of in vivo-labelled PSVs in developing barley endosperm.J Exp Bot. 2014 Jul;65(12):3249-61. doi: 10.1093/jxb/eru175. Epub 2014 May 6. J Exp Bot. 2014. PMID: 24803499 Free PMC article.
-
Endomembrane mediated-trafficking of seed storage proteins: from Arabidopsis to cereal crops.J Exp Bot. 2022 Mar 2;73(5):1312-1326. doi: 10.1093/jxb/erab519. J Exp Bot. 2022. PMID: 34849750 Review.
-
The dynamic behavior of storage organelles in developing cereal seeds and its impact on the production of recombinant proteins.Front Plant Sci. 2014 Sep 3;5:439. doi: 10.3389/fpls.2014.00439. eCollection 2014. Front Plant Sci. 2014. PMID: 25232360 Free PMC article. Review.
Cited by
-
Autophagy during maize endosperm development dampens oxidative stress and promotes mitochondrial clearance.Plant Physiol. 2023 Sep 22;193(2):1395-1415. doi: 10.1093/plphys/kiad340. Plant Physiol. 2023. PMID: 37335933 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

