No changes in hemostasis after COVID-19-heterologous vaccination schedule: A subanalysis of the phase 2 CombiVacS study
- PMID: 36685004
- PMCID: PMC9840220
- DOI: 10.1016/j.rpth.2023.100049
No changes in hemostasis after COVID-19-heterologous vaccination schedule: A subanalysis of the phase 2 CombiVacS study
Abstract
Background: Several cases of unusual thrombotic events and thrombocytopenia were described after vaccination with recombinant adenoviral vectors encoding the spike protein antigen of SARS-CoV-2.
Objectives: The objective of this study was to elucidate the impact of a COVID-19 heterologous vaccination schedule, including priming with adenovirus vaccine, on hemostasis profiles.
Methods: The present study is a subanalysis of the CombiVacS clinical trial initiated in April 2021 that included adult participants previously vaccinated with a single dose of ChAdOx1-S. Between 8 and 12 weeks after vaccination, they were randomly assigned (2:1) to receive either BNT162b2 vaccine (intervention group, n = 99) or continue observation (control group, n = 50). Samples drawn before and 28 days after a vaccination with BNT162b2 were analyzed for platelet count and markers of hemostasis (D-dimer, anti-PF4 antibodies, cfDNA, PAI-1, thrombin generation, and serum capacity to activate platelets).
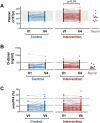
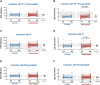
Results: Platelet count from all participants after receiving BNT162b2 was within the normal range. Anti-PF4 antibodies were present in 26% and 18% of the subjects from the control and intervention groups, respectively, at day 28. In most cases, the levels of anti-PF4 antibodies were high before receiving BNT162b2. Serum from these participants did not activate platelets from healthy controls. There were no differences between the groups in PAI-1 and cfDNA plasma levels. According to the D-dimer plasma concentration, the thrombin generation test showed that none of the participants had a procoagulant profile.
Conclusion: Our data suggest that the heterologous vaccination against COVID-19 with ChAdOx1-S and a second dose with BNT162b2 might be safe in terms of haemostasis.
Keywords: COVID-19 vaccine; CombiVacS study; anti-PF4 antibodies; coagulation; hemostasis; heterologous vaccination; thrombocytopenia.
© 2023 The Authors.
Figures


Similar articles
-
Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial.Lancet. 2021 Jul 10;398(10295):121-130. doi: 10.1016/S0140-6736(21)01420-3. Epub 2021 Jun 25. Lancet. 2021. PMID: 34181880 Free PMC article. Clinical Trial.
-
Prevalence of thrombocytopenia, anti-platelet factor 4 antibodies and D-dimer elevation in Thai people After ChAdOx1 nCoV-19 vaccination.Res Pract Thromb Haemost. 2021 Sep 18;5(6):e12580. doi: 10.1002/rth2.12580. eCollection 2021 Aug. Res Pract Thromb Haemost. 2021. PMID: 34568726 Free PMC article.
-
Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine.J Korean Med Sci. 2021 Nov 29;36(46):e311. doi: 10.3346/jkms.2021.36.e311. J Korean Med Sci. 2021. PMID: 34845875 Free PMC article.
-
The role of anti-platelet factor 4 antibodies and platelet activation tests in patients with vaccine-induced immune thrombotic thrombocytopenia: Brief report on a comparison of the laboratory diagnosis and literature review.Clin Chim Acta. 2022 Apr 1;529:42-45. doi: 10.1016/j.cca.2022.02.003. Epub 2022 Feb 12. Clin Chim Acta. 2022. PMID: 35167842 Free PMC article. Review.
-
Potential mechanisms of vaccine-induced thrombosis.Eur J Intern Med. 2022 Nov;105:1-7. doi: 10.1016/j.ejim.2022.08.002. Epub 2022 Aug 8. Eur J Intern Med. 2022. PMID: 35953336 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

