Recurrent visceral leishmaniasis relapses in HIV co-infected patients are characterized by less efficient immune responses and higher parasite load
- PMID: 36685039
- PMCID: PMC9845767
- DOI: 10.1016/j.isci.2022.105867
Recurrent visceral leishmaniasis relapses in HIV co-infected patients are characterized by less efficient immune responses and higher parasite load
Abstract
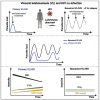
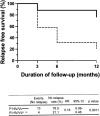
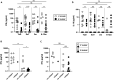
Visceral leishmaniasis (VL) and HIV co-infection (VL/HIV) has emerged as a significant public health problem in Ethiopia, with up to 30% of patients with VL co-infected with HIV. These patients suffer from recurrent VL relapses and increased mortality. Those with a previous history of VL relapses (recurrent VL/HIV) experience increased VL relapses as compared to patients with HIV presenting with their first episode of VL (primary VL/HIV). Our aim was to identify drivers that account for the higher rate of VL relapses in patients with recurrent VL/HIV (n = 28) as compared to primary VL/HIV (n = 21). Our results show that the relapse-free survival in patients with recurrent VL/HIV was shorter, that they had higher parasite load, lower weight gain, and lower recovery of all blood cell lineages. Their poorer prognosis was characterized by lower production of IFN-gamma, lower CD4+ T cell counts, and higher expression of programmed cell death protein 1 (PD1) on T cells.
Keywords: Immune response; Immunology; Virology.
© 2023 The Authors.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures






Similar articles
-
Immunological factors, but not clinical features, predict visceral leishmaniasis relapse in patients co-infected with HIV.Cell Rep Med. 2021 Dec 30;3(1):100487. doi: 10.1016/j.xcrm.2021.100487. eCollection 2022 Jan 18. Cell Rep Med. 2021. PMID: 35106507 Free PMC article.
-
Diversity and Within-Host Evolution of Leishmania donovani from Visceral Leishmaniasis Patients with and without HIV Coinfection in Northern Ethiopia.mBio. 2021 Jun 29;12(3):e0097121. doi: 10.1128/mBio.00971-21. Epub 2021 Jun 29. mBio. 2021. PMID: 34182785 Free PMC article.
-
Impaired Thymic Output Can Be Related to the Low Immune Reconstitution and T Cell Repertoire Disturbances in Relapsing Visceral Leishmaniasis Associated HIV/AIDS Patients.Front Immunol. 2020 May 20;11:953. doi: 10.3389/fimmu.2020.00953. eCollection 2020. Front Immunol. 2020. PMID: 32508833 Free PMC article.
-
The impact of highly active antiretroviral therapy (HAART) on visceral leishmaniasis in Spanish patients who are co-infected with HIV.Ann Trop Med Parasitol. 2003 Oct;97 Suppl 1:143-7. doi: 10.1179/000349803225002615. Ann Trop Med Parasitol. 2003. PMID: 14678641 Review.
-
PKDL and other dermal lesions in HIV co-infected patients with Leishmaniasis: review of clinical presentation in relation to immune responses.PLoS Negl Trop Dis. 2014 Nov 20;8(11):e3258. doi: 10.1371/journal.pntd.0003258. eCollection 2014. PLoS Negl Trop Dis. 2014. PMID: 25412435 Free PMC article. Review.
Cited by
-
In silico approaches supporting drug repurposing for Leishmaniasis: a scoping review.EXCLI J. 2024 Sep 3;23:1117-1169. doi: 10.17179/excli2024-7552. eCollection 2024. EXCLI J. 2024. PMID: 39421030 Free PMC article.
-
Visceral Leishmaniasis-Human Immunodeficiency Virus-Coinfected Patients Are Highly Infectious to Sandflies in an Endemic Area in India.J Infect Dis. 2024 Jun 14;229(6):1909-1912. doi: 10.1093/infdis/jiae156. J Infect Dis. 2024. PMID: 38713583 Free PMC article.
-
Comparison of the Diagnostic Performances of Five Different Tests in Diagnosing Visceral Leishmaniasis in an Endemic Region of Ethiopia.Diagnostics (Basel). 2024 Jan 11;14(2):163. doi: 10.3390/diagnostics14020163. Diagnostics (Basel). 2024. PMID: 38248040 Free PMC article.
-
An integrated analysis of the structural changes and gene expression of spleen in human visceral leishmaniasis with and without HIV coinfection.PLoS Negl Trop Dis. 2024 Jun 6;18(6):e0011877. doi: 10.1371/journal.pntd.0011877. eCollection 2024 Jun. PLoS Negl Trop Dis. 2024. PMID: 38843306 Free PMC article.
-
Persistent T cell unresponsiveness associated with chronic visceral leishmaniasis in HIV-coinfected patients.Commun Biol. 2024 May 3;7(1):524. doi: 10.1038/s42003-024-06225-2. Commun Biol. 2024. PMID: 38702419 Free PMC article.
References
-
- Ruiz-Postigo J.A., Grout L., Jain S. Global leishmaniasis surveillance, 2017–2018, and first report on 5 additional indicators. Wkly. Epidemiol. Rec. 2020;25:165–280.
-
- WHO Technical Report Series Control of the Leishmaniasis. 2010. http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf - PubMed
-
- Diro E., Edwards T., Ritmeijer K., Fikre H., Abongomera C., Kibret A., Bardonneau C., Soipei P., Mutinda B., Omollo R., et al. Long term outcomes and prognostics of visceral leishmaniasis in HIV infected patients with use of pentamidine as secondary prophylaxis based on CD4 level: a prospective cohort study in Ethiopia. PLoS Neglected Trop. Dis. 2019;13:e0007132. doi: 10.1371/journal.pntd.0007132. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

