Interleukin 11 confers resistance to dextran sulfate sodium-induced colitis in mice
- PMID: 36685040
- PMCID: PMC9852934
- DOI: 10.1016/j.isci.2023.105934
Interleukin 11 confers resistance to dextran sulfate sodium-induced colitis in mice
Abstract
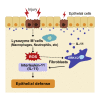
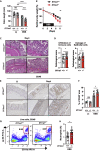
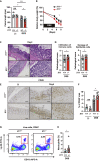
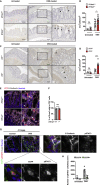
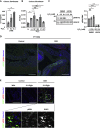
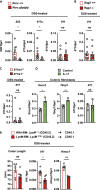
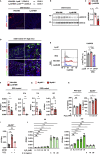
Intestinal homeostasis is tightly regulated by epithelial cells, leukocytes, and stromal cells, and its dysregulation is associated with inflammatory bowel diseases. Interleukin (IL)-11, a member of the IL-6 family of cytokines, is produced by inflammatory fibroblasts during acute colitis. However, the role of IL-11 in the development of colitis is still unclear. Herein, we showed that IL-11 ameliorated DSS-induced acute colitis in mouse models. We found that deletion of Il11ra1 or Il11 rendered mice highly susceptible to DSS-induced colitis compared to the respective control mice. The number of apoptotic epithelial cells was increased in DSS-treated Il11ra1- or Il11-deficient mice. Moreover, we showed that IL-11 production was regulated by reactive oxygen species (ROS) produced by lysozyme M-positive myeloid cells. These findings indicate that fibroblast-produced IL-11 plays an important role in protecting the mucosal epithelium in acute colitis. Myeloid cell-derived ROS contribute to the attenuation of colitis through the production of IL-11.
Keywords: Components of the immune system; Immunology; Molecular physiology.
© 2023 The Authors.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

