The calcineurin regulator Sarah enables distinct forms of homeostatic plasticity at the Drosophila neuromuscular junction
- PMID: 36685082
- PMCID: PMC9846150
- DOI: 10.3389/fnsyn.2022.1033743
The calcineurin regulator Sarah enables distinct forms of homeostatic plasticity at the Drosophila neuromuscular junction
Abstract
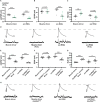
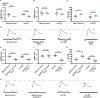
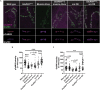
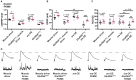
Introduction: The ability of synapses to maintain physiological levels of evoked neurotransmission is essential for neuronal stability. A variety of perturbations can disrupt neurotransmission, but synapses often compensate for disruptions and work to stabilize activity levels, using forms of homeostatic synaptic plasticity. Presynaptic homeostatic potentiation (PHP) is one such mechanism. PHP is expressed at the Drosophila melanogaster larval neuromuscular junction (NMJ) synapse, as well as other NMJs. In PHP, presynaptic neurotransmitter release increases to offset the effects of impairing muscle transmitter receptors. Prior Drosophila work has studied PHP using different ways to perturb muscle receptor function-either acutely (using pharmacology) or chronically (using genetics). Some of our prior data suggested that cytoplasmic calcium signaling was important for expression of PHP after genetic impairment of glutamate receptors. Here we followed up on that observation. Methods: We used a combination of transgenic Drosophila RNA interference and overexpression lines, along with NMJ electrophysiology, synapse imaging, and pharmacology to test if regulators of the calcium/calmodulin-dependent protein phosphatase calcineurin are necessary for the normal expression of PHP. Results: We found that either pre- or postsynaptic dysregulation of a Drosophila gene regulating calcineurin, sarah (sra), blocks PHP. Tissue-specific manipulations showed that either increases or decreases in sra expression are detrimental to PHP. Additionally, pharmacologically and genetically induced forms of expression of PHP are functionally separable depending entirely upon which sra genetic manipulation is used. Surprisingly, dual-tissue pre- and postsynaptic sra knockdown or overexpression can ameliorate PHP blocks revealed in single-tissue experiments. Pharmacological and genetic inhibition of calcineurin corroborated this latter finding. Discussion: Our results suggest tight calcineurin regulation is needed across multiple tissue types to stabilize peripheral synaptic outputs.
Keywords: Drosophila melanogaster; NMJ–neuromuscular junction; Sarah; calcineurin (CaN); neurotransmission; plasticity; synaptic dysfunction; synaptic homeostasis.
Copyright © 2023 Armstrong and Frank.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
C-terminal Src Kinase Gates Homeostatic Synaptic Plasticity and Regulates Fasciclin II Expression at the Drosophila Neuromuscular Junction.PLoS Genet. 2016 Feb 22;12(2):e1005886. doi: 10.1371/journal.pgen.1005886. eCollection 2016 Feb. PLoS Genet. 2016. PMID: 26901416 Free PMC article.
-
The Maintenance of Synaptic Homeostasis at the Drosophila Neuromuscular Junction Is Reversible and Sensitive to High Temperature.eNeuro. 2017 Dec 12;4(6):ENEURO.0220-17.2017. doi: 10.1523/ENEURO.0220-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29255795 Free PMC article.
-
Eliciting Presynaptic Homeostatic Potentiation at the Drosophila Larval Neuromuscular Junction.Cold Spring Harb Protoc. 2025 May 5;2025(5):pdb.prot108424. doi: 10.1101/pdb.prot108424. Cold Spring Harb Protoc. 2025. PMID: 38688541
-
Homeostatic plasticity at the Drosophila neuromuscular junction.Neuropharmacology. 2014 Mar;78:63-74. doi: 10.1016/j.neuropharm.2013.06.015. Epub 2013 Jun 24. Neuropharmacology. 2014. PMID: 23806804 Free PMC article. Review.
-
Synaptic homeostats: latent plasticity revealed at the Drosophila neuromuscular junction.Cell Mol Life Sci. 2021 Apr;78(7):3159-3179. doi: 10.1007/s00018-020-03732-3. Epub 2021 Jan 15. Cell Mol Life Sci. 2021. PMID: 33449150 Free PMC article. Review.
Cited by
-
Using Electrophysiology to Study Homeostatic Plasticity at the Drosophila Neuromuscular Junction.Cold Spring Harb Protoc. 2025 May 5;2025(5):pdb.top108393. doi: 10.1101/pdb.top108393. Cold Spring Harb Protoc. 2025. PMID: 38688539
-
Versatile Endogenous Editing of GluRIIA in Drosophila melanogaster.Cells. 2024 Feb 10;13(4):323. doi: 10.3390/cells13040323. Cells. 2024. PMID: 38391936 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

