Mutant plasminogen in hereditary angioedema is bypassing FXII/kallikrein to generate bradykinin
- PMID: 36685169
- PMCID: PMC9849239
- DOI: 10.3389/fphys.2022.1090732
Mutant plasminogen in hereditary angioedema is bypassing FXII/kallikrein to generate bradykinin
Abstract
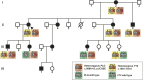
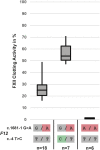
Hereditary angioedema (HAE) is characterized by recurrent localized edema in various organs, which can be potentially fatal. There are different types of hereditary angioedema, which include genetic deficiency of C1 inhibitor (C1-INH) and hereditary angioedema with normal C1-INH (HAEnCI). In HAEnCI patients mutations have been identified in the F12, PLG, KNG1, ANGPT1, MYOF, and HS3ST6 genes. The release of bradykinin from kininogen via the kallikrein-kinin system (KKS) has been shown to be the main mediator in HAE-FXII, but for HAE-PLG there are only first indications how the PLG mutations can result in bradykinin release. Here we identified in a multi-generation HAE-PLG family an additional F12 mutation, resulting in the loss of one F12 allele. There were no differences in the clinical presentation between HAE-PLG patients with and without the additional F12 mutation, thus we concluded that the kallikrein-kinin system is bypassed in HAE-PLG. Structural modeling and in vitro assays using purified proteins confirmed the PLG mutation c.988A>G; p.K330E to be a gain of function mutation resulting in an increased bradykinin release by direct cleavage of high molecular weight kininogen (HMWK). Thus, we can provide clinical and experimental evidence that mutant plasminogen in HAE-PLG is bypassing FXII/kallikrein to generate bradykinin.
Keywords: FXII; HAE-PLG; Hereditary angioedema (HAE); bradykinin; kallikrein-kinin system (KKS); normal C1-INH; plasminogen.
Copyright © 2023 Hintze, Möhl, Beyerl, Wulff, Wieser, Bork and Meinke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

