Bioengineered silkworm model for expressing human neurotrophin-4 with potential biomedical application
- PMID: 36685209
- PMCID: PMC9846172
- DOI: 10.3389/fphys.2022.1104929
Bioengineered silkworm model for expressing human neurotrophin-4 with potential biomedical application
Abstract
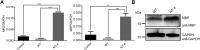
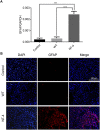
Neurotrophin-4 (NT-4) is a neurotrophic factor that plays important roles in maintaining nerve cell survival, regulating neuronal differentiation and apoptosis, and promoting nerve injury repair. However, the source of sufficient NT-4 protein and efficient delivery of NT-4 remain a challenge. This study aims to express an activated human NT-4 protein in a large scale by genetically engineering silk gland bioreactor of silkworm as a host. We showed that the expression of human NT-4-functionalized silk material could promote proliferation of mouse HT22 cells when compared to the natural silk protein, and no obvious cytotoxicity was observed under the conditions of different silk materials. Importantly, this functional silk material was able to induce the potential differentiation of HT22 cells, promote peripheral neural cell migration and neurite outgrowth of chicken embryo dorsal root ganglion (DRG). All these results demonstrated a high bioactivity of human NT-4 protein produced in silk gland. Therefore, based on the silkworm model, the further fabrication of different silk materials-carrying active NT-4 protein with good mechanical properties and great biocompatibility will give promising applications in tissue engineering and neurons regeneration.
Keywords: biomedical application; bombyx mori; human neurotrophic-4; silk gland bioreactor; silk material.
Copyright © 2023 Zhang, Li, Lan, Guo, Chen, Wang, Shen, Xia and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



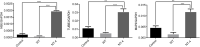

Similar articles
-
Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications.Insects. 2025 Jun 27;16(7):676. doi: 10.3390/insects16070676. Insects. 2025. PMID: 40725307 Free PMC article.
-
Transgenic silkworm expressing bioactive human ciliary neurotrophic factor for biomedical application.Insect Sci. 2025 Jun;32(3):809-820. doi: 10.1111/1744-7917.13442. Epub 2024 Sep 1. Insect Sci. 2025. PMID: 39219303
-
Fabrication of the FGF1-functionalized sericin hydrogels with cell proliferation activity for biomedical application using genetically engineered Bombyx mori (B. mori) silk.Acta Biomater. 2018 Oct 1;79:239-252. doi: 10.1016/j.actbio.2018.08.031. Epub 2018 Aug 25. Acta Biomater. 2018. PMID: 30149211
-
Silk-based biomaterials.Biomaterials. 2003 Feb;24(3):401-16. doi: 10.1016/s0142-9612(02)00353-8. Biomaterials. 2003. PMID: 12423595 Review.
-
The advances and perspectives of recombinant protein production in the silk gland of silkworm Bombyx mori.Transgenic Res. 2014 Oct;23(5):697-706. doi: 10.1007/s11248-014-9826-8. Epub 2014 Aug 12. Transgenic Res. 2014. PMID: 25113390 Review.
Cited by
-
Biosynthesis of Bioactive Human Neurotrophic Factor 3 in Silkworms and Its Biomedical Applications.Insects. 2025 Jun 27;16(7):676. doi: 10.3390/insects16070676. Insects. 2025. PMID: 40725307 Free PMC article.
-
High mechanical property silk produced by transgenic silkworms expressing the Drosophila Dumpy.Front Bioeng Biotechnol. 2024 Feb 12;12:1359587. doi: 10.3389/fbioe.2024.1359587. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38410165 Free PMC article.
-
Modifying Naturally Occurring, Nonmammalian-Sourced Biopolymers for Biomedical Applications.ACS Biomater Sci Eng. 2024 Oct 14;10(10):5915-5938. doi: 10.1021/acsbiomaterials.4c00689. Epub 2024 Sep 11. ACS Biomater Sci Eng. 2024. PMID: 39259773 Review.
References
-
- Alexander J. E., Hunt D. F., Lee M. K., Shabanowitz J., Michel H., Berlin S. C., et al. (1991). Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 88 (11), 4685–4689. 10.1073/pnas.88.11.4685 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

