Multiple aspects of lymphatic dysfunction in an ApoE -/- mouse model of hypercholesterolemia
- PMID: 36685213
- PMCID: PMC9852907
- DOI: 10.3389/fphys.2022.1098408
Multiple aspects of lymphatic dysfunction in an ApoE -/- mouse model of hypercholesterolemia
Abstract
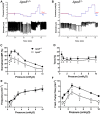
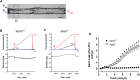
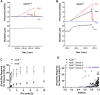
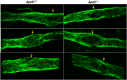
Introduction: Rodent models of cardiovascular disease have uncovered various types of lymphatic vessel dysfunction that occur in association with atherosclerosis, type II diabetes and obesity. Previously, we presented in vivo evidence for impaired lymphatic drainage in apolipoprotein E null (ApoE -/- ) mice fed a high fat diet (HFD). Whether this impairment relates to the dysfunction of collecting lymphatics remains an open question. The ApoE -/- mouse is a well-established model of cardiovascular disease, in which a diet rich in fat and cholesterol on an ApoE deficient background accelerates the development of hypercholesteremia, atherosclerotic plaques and inflammation of the skin and other tissues. Here, we investigated various aspects of lymphatic function using ex vivo tests of collecting lymphatic vessels from ApoE +/+ or ApoE -/- mice fed a HFD. Methods: Popliteal collectors were excised from either strain and studied under defined conditions in which we could quantify changes in lymphatic contractile strength, lymph pump output, secondary valve function, and collecting vessel permeability. Results: Our results show that all these aspects of lymphatic vessel function are altered in deleterious ways in this model of hypercholesterolemia. Discussion: These findings extend previous in vivo observations suggesting significant dysfunction of lymphatic endothelial cells and smooth muscle cells from collecting vessels in association with a HFD on an ApoE-deficient background. An implication of our study is that collecting vessel dysfunction in this context may negatively impact the removal of cholesterol by the lymphatic system from the skin and the arterial wall and thereby exacerbate the progression and/or severity of atherosclerosis and associated inflammation.
Keywords: back-leak; contractile dysfunction; high fat diet; lymphatic endothelium; lymphatic muscle; lymphatic valve; permeability; pump limit.
Copyright © 2023 Davis, Scallan, Castorena-Gonzalez, Kim, Ying, Pin and Angeli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agabiti-Rosei C., Paini A., De Ciuceis C., Withers S., Greenstein A., Heagerty A. M., et al. (2018). Modulation of vascular reactivity by perivascular adipose tissue (PVAT). Curr. Hypertens. Rep. 20, 44. - PubMed
-
- Berendam S. J., Koeppel A. F., Godfrey N. R., Rouhani S. J., Woods A. N., Rodriguez A. B., et al. (2019). Comparative transcriptomic analysis identifies a range of immunologically related functional elaborations of lymph node associated lymphatic and blood endothelial cells. Front. Immunol. 10, 816. 10.3389/fimmu.2019.00816 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

