Expression, purification, and functional characterization of soluble recombinant full-length simian immunodeficiency virus (SIV) Pr55Gag
- PMID: 36685375
- PMCID: PMC9853374
- DOI: 10.1016/j.heliyon.2023.e12892
Expression, purification, and functional characterization of soluble recombinant full-length simian immunodeficiency virus (SIV) Pr55Gag
Abstract
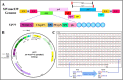
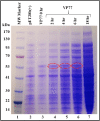
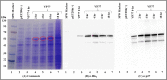
The simian immunodeficiency virus (SIV) precursor polypeptide Pr55Gag drives viral assembly and facilitates specific recognition and packaging of the SIV genomic RNA (gRNA) into viral particles. While several studies have tried to elucidate the role of SIV Pr55Gag by expressing its different components independently, studies using full-length SIV Pr55Gag have not been conducted, primarily due to the unavailability of purified and biologically active full-length SIV Pr55Gag. We successfully expressed soluble, full-length SIV Pr55Gag with His6-tag in bacteria and purified it using affinity and gel filtration chromatography. In the process, we identified within Gag, a second in-frame start codon downstream of a putative Shine-Dalgarno-like sequence resulting in an additional truncated form of Gag. Synonymously mutating this sequence allowed expression of full-length Gag in its native form. The purified Gag assembled into virus-like particles (VLPs) in vitro in the presence of nucleic acids, revealing its biological functionality. In vivo experiments also confirmed formation of functional VLPs, and quantitative reverse transcriptase PCR demonstrated efficient packaging of SIV gRNA by these VLPs. The methodology we employed ensured the availability of >95% pure, biologically active, full-length SIV Pr55Gag which should facilitate future studies to understand protein structure and RNA-protein interactions involved during SIV gRNA packaging.
Keywords: Chromatography; In vitro and in vivo viral particle assembly; Protein purification and expression; RNA binding protein; RNA packaging; Retroviruses; SIV Pr55Gag His6-tagged fusion protein purification; Simian immunodeficiency virus (SIV).
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag.Viruses. 2019 Jul 27;11(8):689. doi: 10.3390/v11080689. Viruses. 2019. PMID: 31357656 Free PMC article.
-
The C-terminal p6 domain of the HIV-1 Pr55Gag precursor is required for specific binding to the genomic RNA.RNA Biol. 2018;15(7):923-936. doi: 10.1080/15476286.2018.1481696. Epub 2018 Aug 4. RNA Biol. 2018. PMID: 29954247 Free PMC article.
-
Biochemical and Functional Characterization of Mouse Mammary Tumor Virus Full-Length Pr77Gag Expressed in Prokaryotic and Eukaryotic Cells.Viruses. 2018 Jun 18;10(6):334. doi: 10.3390/v10060334. Viruses. 2018. PMID: 29912170 Free PMC article.
-
The Life-Cycle of the HIV-1 Gag-RNA Complex.Viruses. 2016 Sep 10;8(9):248. doi: 10.3390/v8090248. Viruses. 2016. PMID: 27626439 Free PMC article. Review.
-
On the Selective Packaging of Genomic RNA by HIV-1.Viruses. 2016 Sep 12;8(9):246. doi: 10.3390/v8090246. Viruses. 2016. PMID: 27626441 Free PMC article. Review.
Cited by
-
MMTV RNA packaging requires an extended long-range interaction for productive Gag binding to packaging signals.PLoS Biol. 2024 Oct 3;22(10):e3002827. doi: 10.1371/journal.pbio.3002827. eCollection 2024 Oct. PLoS Biol. 2024. PMID: 39361708 Free PMC article.
References
-
- Skalka A.M. 2018. Discovering Retroviruses : Beacons in the Biosphere.
LinkOut - more resources
Full Text Sources

