A novel model of urosepsis in rats developed by injection of Escherichia coli into the renal pelvis
- PMID: 36685507
- PMCID: PMC9849364
- DOI: 10.3389/fimmu.2022.1074488
A novel model of urosepsis in rats developed by injection of Escherichia coli into the renal pelvis
Abstract
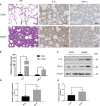
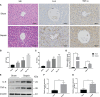
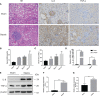
Despite extensive research, urosepsis remains a life-threatening, high-mortality disease. Currently, animal models of urosepsis widely accepted by investigators are very scarce. This study aimed to establish a standardized and reproducible model of urosepsis in rats. Forty adult Wistar rats were randomly divided into four groups according to the concentration of injected E. coli suspensions: Sham, Sep 3×, Sep 6×, and Sep 12×. Because the ureter is so thin and fragile, no conventional needle can be inserted into the ureter, which is probably why rats are rarely used to develop models of urosepsis. To solve this problem, the left ureter was ligated in the first procedure. After 24 hours, the left ureter above the ligation was significantly dilated, then saline or different concentrations of E. coli at 3 ml/kg were injected into the left renal pelvis using a 30G needle. The left ureter was subsequently ligated again at a distance of 1 cm from the renal hilum to maintain high pressure in the renal pelvis. Following injection of E. coli or saline for 24 h, three rats from each group were sacrificed and their organs (lung, liver, and right kidney) were collected. In contrast, the remaining seven rats continued to be observed for survival. At 10 days after E. coli injection, rats in the sep12× group had a higher mortality rate (100%) compared to the sep3× group (28.6%) or the sep6× group (71.4%). The significant changes in peripheral blood WBC count, serum IL-6 and TNF-α levels were also in the sep12× group. In addition, rats in the sepsis group showed multi-organ dysfunction, including damage to the lungs, liver, and kidneys. The establishment of a standardized rat model of urosepsis may be of great value for studying the pathophysiological of urosepsis.
Keywords: Escherichia coli; animal model; pathophysiology; rats; upper urinary tract obstruction; urosepsis.
Copyright © 2023 Cao, Bai, Si, Yan, Zhang, Yisha, Lu, Tuoheti, Guo, Chen, Bai and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A novel model of urosepsis in mice developed by ureteral ligation and injection of Escherichia coli into the renal pelvis.Heliyon. 2024 Feb 1;10(3):e25522. doi: 10.1016/j.heliyon.2024.e25522. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38327418 Free PMC article.
-
The Early Diagnostic Efficacy of Serum Histone H3 in Rabbit Urosepsis Model.Biomed Res Int. 2021 Jul 16;2021:9969344. doi: 10.1155/2021/9969344. eCollection 2021. Biomed Res Int. 2021. Retraction in: Biomed Res Int. 2024 Mar 20;2024:9835161. doi: 10.1155/2024/9835161. PMID: 34327242 Free PMC article. Retracted.
-
Early and rapid prediction of postoperative infections following percutaneous nephrolithotomy in patients with complex kidney stones.BJU Int. 2019 Jun;123(6):1041-1047. doi: 10.1111/bju.14484. Epub 2018 Aug 9. BJU Int. 2019. PMID: 30007112
-
Virulence factors and O groups of Escherichia coli strains isolated from cultures of blood specimens from urosepsis and non-urosepsis patients.Microbiologia. 1994 Sep;10(3):249-56. Microbiologia. 1994. PMID: 7532950 Review.
-
Pharmacokinetic characteristics of antimicrobials and optimal treatment of urosepsis.Clin Pharmacokinet. 2007;46(4):291-305. doi: 10.2165/00003088-200746040-00003. Clin Pharmacokinet. 2007. PMID: 17375981 Review.
Cited by
-
TR4 worsen urosepsis by regulating GSDMD.Eur J Med Res. 2024 Mar 1;29(1):151. doi: 10.1186/s40001-024-01742-6. Eur J Med Res. 2024. PMID: 38429762 Free PMC article.
-
Dynamics of Urinary Extracellular DNA in Urosepsis.Biomolecules. 2023 Jun 17;13(6):1008. doi: 10.3390/biom13061008. Biomolecules. 2023. PMID: 37371588 Free PMC article.
-
A novel model of urosepsis in mice developed by ureteral ligation and injection of Escherichia coli into the renal pelvis.Heliyon. 2024 Feb 1;10(3):e25522. doi: 10.1016/j.heliyon.2024.e25522. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38327418 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

