The roles of long noncoding RNA-mediated macrophage polarization in respiratory diseases
- PMID: 36685535
- PMCID: PMC9849253
- DOI: 10.3389/fimmu.2022.1110774
The roles of long noncoding RNA-mediated macrophage polarization in respiratory diseases
Abstract
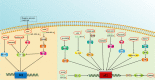
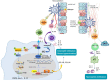
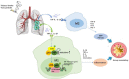
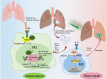
Macrophages play an essential role in maintaining the normal function of the innate and adaptive immune responses during host defence. Macrophages acquire diverse functional phenotypes in response to various microenvironmental stimuli, and are mainly classified into classically activated macrophages (M1) and alternatively activated macrophages (M2). Macrophage polarization participates in the inflammatory, fibrotic, and oncogenic processes of diverse respiratory diseases by changing phenotype and function. In recent decades, with the advent of broad-range profiling methods such as microarrays and next-generation sequencing, the discovery of RNA transcripts that do not encode proteins termed "noncoding RNAs (ncRNAs)" has become more easily accessible. As one major member of the regulatory ncRNA family, long noncoding RNAs (lncRNAs, transcripts >200 nucleotides) participate in multiple pathophysiological processes, including cell proliferation, differentiation, and apoptosis, and vary with different stimulants and cell types. Emerging evidence suggests that lncRNAs account for the regulation of macrophage polarization and subsequent effects on respiratory diseases. In this review, we summarize the current published literature from the PubMed database concerning lncRNAs relevant to macrophage polarization and the underlying molecular mechanisms during the occurrence and development of respiratory diseases. These differentially expressed lncRNAs are expected to be biomarkers and targets for the therapeutic regulation of macrophage polarization during disease development.
Keywords: M1/M2 polarization; long noncoding RNAs; lung cancer; macrophages; respiratory diseases.
Copyright © 2023 Qiao, Ding, Wu, Zhang, Yin, Wang, Wang and Kang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
-
- WHO . Coronovirus (COVID-19) dashboard. World Health Organization; (Accessed February 9, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

