Prognostic factors of second hematopoietic allogeneic stem cell transplantation among hematological malignancy patients relapsed after first hematopoietic stem cell transplantation: A single center study
- PMID: 36685540
- PMCID: PMC9846785
- DOI: 10.3389/fimmu.2022.1066748
Prognostic factors of second hematopoietic allogeneic stem cell transplantation among hematological malignancy patients relapsed after first hematopoietic stem cell transplantation: A single center study
Abstract
Introduction: We aimed to evaluate prognostic factors of a second allogeneic stem cell transplantation (allo-HSCT2) among hematological malignancy patients who have relapsed after the first allo-HSCT(allo-HSCT1).
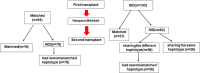
Methods: We retrospectively analyzed 199 hematological malignancy patients who received allo-HSCT2 as a salvage treatment post allo-HSCT1 relapse between November 2012 and October 2021.
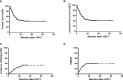
Results: The median age at allo-HSCT2 was 23 (range: 3-60) years. The median time to relapse after HSCT1 was 9 (range: 1-72) months. Prior to allo-HSCT2, patients had the following hematopoietic cell transplantation-comorbidity indexes (HCT-CI): 127 with a score of 0, 52 with a score of 1, and 20 with a score of 2 or greater. Fifty percent of patients received chimeric antigen receptor (CAR) T-cell therapy following HSCT1 relapse. Disease status was minimal residual disease (MRD)-negative complete remission (CR) among 119 patients, MRD-positive CR among 37 patients and non-remission (NR) for 43 patients prior to allo-HSCT2. Allo-HSCT2 was performed from a new donor in 194 patients (97.4%) and 134 patients (67.3%) received a graft with a new mismatched haplotype. The median follow-up time was 24 months (range: 6-98 months), and the 2-year OS and LFS were 43.8% ± 4.0% and 42.1% ± 4.1%, respectively. The 2-year cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) was 30.0%±4.8% and 38.5%±3.8%, respectively. Cox regression multivariate analysis showed that disease statusof MRD-negative CR, HCT-CI score of 0 prior to allo-HSCT2, and new mismatched haplotype donor were predictive factors of improved OS and LFS compared to patients without these characteristics. Based on these three favorable factors, we developed a predictive scoring system for patients who received allo-HSCT2. Patients with a prognostic score of 3 who had the three factors showed a superior 2-year OS of 63.3% ± 6.7% and LFS of 63.3% ± 6.7% and a lower CIR of 5.5% ± 3.1% than patients with a prognostic score of 0. Allo-HSCT2 is feasible and patients with good prognostic features prior to allo-HSCT2 -disease status of CR/MRD- and HCT-CI score of 0 as well as a second donor with a new mismatched haplotype could have the maximal benefit from the second allo-HSCT.
Conclusions: Allo-HSCT2 is feasible and patients with good prognostic features prior to allo-HSCT2 -disease status of CR/MRD- and HCT-CI score of 0 as well as a second donor with a new mismatched haplotype could have the maximal benefit from the second allo-HSCT.
Keywords: first allogeneic hematopoietic stem cell transplantation; hematological malignancy; prognosis factor; relapse; second allogeneic hematopoietic stem cell transplant.
Copyright © 2023 Lu, Zhang, Zhao, Xiong, Sun, Cao, Wei, Zhou, Liu, Yang, Zhang, Lu and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Advances in second hematopoietic stem cell transplantation.Front Immunol. 2024 Jul 9;15:1428101. doi: 10.3389/fimmu.2024.1428101. eCollection 2024. Front Immunol. 2024. PMID: 39044835 Free PMC article. Review.
-
[Efficacy and safety of secondary allogeneic hematopoietic stem cell transplantation in 70 patients with recurrent hematologic malignancies after transplantation].Zhonghua Xue Ye Xue Za Zhi. 2023 Jun 14;44(6):458-464. doi: 10.3760/cma.j.issn.0253-2727.2023.06.003. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 37550200 Free PMC article. Chinese.
-
[Second allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning and donor changes in relapsed hematological malignancies after the first allogeneic transplant].Zhonghua Xue Ye Xue Za Zhi. 2023 Jun 14;44(6):465-471. doi: 10.3760/cma.j.issn.0253-2727.2023.06.004. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 37550201 Free PMC article. Chinese.
-
Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change.J Clin Oncol. 2013 Sep 10;31(26):3259-71. doi: 10.1200/JCO.2012.44.7961. Epub 2013 Aug 5. J Clin Oncol. 2013. PMID: 23918951
-
Efficacy of a Second Allogeneic Hematopoietic Cell Transplant in Relapsed Acute Myeloid Leukemia: Results of a Systematic Review and Meta-Analysis.Transplant Cell Ther. 2022 Nov;28(11):767.e1-767.e11. doi: 10.1016/j.jtct.2022.08.008. Epub 2022 Aug 13. Transplant Cell Ther. 2022. PMID: 35970301
Cited by
-
Post-transplant changes in physical functioning and quality of life in patients undergoing two allogeneic hematopoietic stem cell transplants.Int J Hematol. 2025 Jul 5. doi: 10.1007/s12185-025-04030-z. Online ahead of print. Int J Hematol. 2025. PMID: 40616729
-
How risky is a second allogeneic stem cell transplantation?Leukemia. 2024 Aug;38(8):1799-1807. doi: 10.1038/s41375-024-02318-3. Epub 2024 Jun 25. Leukemia. 2024. PMID: 38918561 Free PMC article.
-
Analysis benefits of a second Allo-HSCT after CAR-T cell therapy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia who relapsed after transplant.Front Immunol. 2023 Jul 4;14:1191382. doi: 10.3389/fimmu.2023.1191382. eCollection 2023. Front Immunol. 2023. PMID: 37469510 Free PMC article.
-
Advances in second hematopoietic stem cell transplantation.Front Immunol. 2024 Jul 9;15:1428101. doi: 10.3389/fimmu.2024.1428101. eCollection 2024. Front Immunol. 2024. PMID: 39044835 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

