Differential regulation of lineage-determining transcription factor expression in innate lymphoid cell and adaptive T helper cell subsets
- PMID: 36685550
- PMCID: PMC9846361
- DOI: 10.3389/fimmu.2022.1081153
Differential regulation of lineage-determining transcription factor expression in innate lymphoid cell and adaptive T helper cell subsets
Abstract
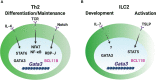
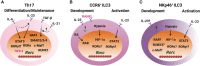
CD4 T helper (Th) cell subsets, including Th1, Th2 and Th17 cells, and their innate counterparts innate lymphoid cell (ILC) subsets consisting of ILC1s, ILC2s and ILC3s, display similar effector cytokine-producing capabilities during pro-inflammatory immune responses. These lymphoid cell subsets utilize the same set of lineage-determining transcription factors (LDTFs) for their differentiation, development and functions. The distinct ontogeny and developmental niches between Th cells and ILCs indicate that they may adopt different external signals for the induction of LDTF during lineage commitment. Increasing evidence demonstrates that many conserved cis-regulatory elements at the gene loci of LDTFs are often preferentially utilized for the induction of LDTF expression during Th cell differentiation and ILC development at different stages. In this review, we discuss the functions of lineage-related cis-regulatory elements in inducing T-bet, GATA3 or RORγt expression based on the genetic evidence provided in recent publications. We also review and compare the upstream signals involved in LDTF induction in Th cells and ILCs both in vitro and in vivo. Finally, we discuss the possible mechanisms and physiological importance of regulating LDTF dynamic expression during ILC development and activation.
Keywords: CD4 T helper cells; ILC development; Th cell differentiation; epigenetic modification; innate lymphoid cells; lineage-determining transcription factor.
Copyright © 2023 Fang, Healy and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Distinct regulatory machineries underlying divergent chromatin landscapes distinguish innate lymphoid cells from T helper cells.Front Immunol. 2023 Dec 1;14:1271879. doi: 10.3389/fimmu.2023.1271879. eCollection 2023. Front Immunol. 2023. PMID: 38106414 Free PMC article.
-
Transcriptional regulation of adaptive and innate lymphoid lineage specification.Immunol Rev. 2021 Mar;300(1):65-81. doi: 10.1111/imr.12935. Epub 2020 Dec 7. Immunol Rev. 2021. PMID: 33615514 Review.
-
CD4 T Helper Cell Subsets and Related Human Immunological Disorders.Int J Mol Sci. 2020 Oct 28;21(21):8011. doi: 10.3390/ijms21218011. Int J Mol Sci. 2020. PMID: 33126494 Free PMC article. Review.
-
GATA3 Regulates the Development and Functions of Innate Lymphoid Cell Subsets at Multiple Stages.Front Immunol. 2017 Nov 14;8:1571. doi: 10.3389/fimmu.2017.01571. eCollection 2017. Front Immunol. 2017. PMID: 29184556 Free PMC article. Review.
-
Lymphoid tissue inducer-A divergent member of the ILC family.Cytokine Growth Factor Rev. 2018 Aug;42:5-12. doi: 10.1016/j.cytogfr.2018.02.004. Epub 2018 Feb 13. Cytokine Growth Factor Rev. 2018. PMID: 29454785 Free PMC article. Review.
Cited by
-
A distal enhancer of GATA3 regulates Th2 differentiation and allergic inflammation.Proc Natl Acad Sci U S A. 2024 Jul 2;121(27):e2320727121. doi: 10.1073/pnas.2320727121. Epub 2024 Jun 26. Proc Natl Acad Sci U S A. 2024. PMID: 38923989 Free PMC article.
-
The kinase ITK controls a Ca2+-mediated switch that balances TH17 and Treg cell differentiation.Sci Signal. 2024 Jul 23;17(846):eadh2381. doi: 10.1126/scisignal.adh2381. Epub 2024 Jul 23. Sci Signal. 2024. PMID: 39042726 Free PMC article.
-
Trichloroethylene metabolite modulates DNA methylation-dependent gene expression in Th1-polarized CD4+ T cells from autoimmune-prone mice.Toxicol Sci. 2024 May 28;199(2):289-300. doi: 10.1093/toxsci/kfae032. Toxicol Sci. 2024. PMID: 38518092 Free PMC article.
-
Thymic dendritic cell-derived IL-27p28 promotes the establishment of functional bias against IFN-γ production in newly generated CD4+ T cells through STAT1-related epigenetic mechanisms.Elife. 2025 May 14;13:RP96868. doi: 10.7554/eLife.96868. Elife. 2025. PMID: 40366856 Free PMC article.
-
Distinct regulatory machineries underlying divergent chromatin landscapes distinguish innate lymphoid cells from T helper cells.Front Immunol. 2023 Dec 1;14:1271879. doi: 10.3389/fimmu.2023.1271879. eCollection 2023. Front Immunol. 2023. PMID: 38106414 Free PMC article.
References
-
- Sun B. Advances in experimental medicine and biology. Dordrecht: Springer Netherlands : Imprint: Springer; (2014). p. 1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

