National experience with adenosine deaminase deficiency related SCID in Polish children
- PMID: 36685585
- PMCID: PMC9853035
- DOI: 10.3389/fimmu.2022.1058623
National experience with adenosine deaminase deficiency related SCID in Polish children
Abstract
Introduction: Deficiency of adenosine deaminase (ADA) manifests as severe combined immunodeficiency (SCID), caused by accumulation of toxic purine degradation by-products. Untreated patients develop immune and non-immune symptoms with fatal clinical course. According to ESID and EBMT recommendations enzyme replacement therapy (ERT) should be implemented as soon as possible to stabilize the patient's general condition, normalize transaminases, treat pulmonary proteinosis, bone dysplasia, and protect from neurological damage. Hematopoietic stem cell transplantation (HSCT) from a matched related donor (MRD) is a treatment of choice. In absence of such donor, gene therapy (GT) should be considered. HSCT from a matched unrelated donor (MUD) and haploidentical hematopoietic stem cell transplantation (hHSCT) are associated with worse prognosis.
Material and methods: We retrospectively evaluated the clinical course and results of biochemical, immunological and genetic tests of 7 patients diagnosed in Poland with ADA deficiency since 2010 to 2022.
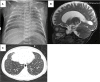
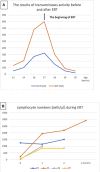
Results: All patients demonstrated lymphopenia affecting of T, B and NK cells. Diagnosis was made on the basis of ADA activity in red blood cells and/or genetic testing. Patients manifested with various non-immunological symptoms including: lung proteinosis, skeletal dysplasia, liver dysfunction, atypical hemolytic-uremic syndrome, and psychomotor development disorders. Five patients underwent successful HSCT: 3 patients from matched unrelated donor, 2 from matched sibling donor, and 1 haploidentical from a parental donor. In 4 patients HSCT was preceded by enzyme therapy (lasting from 2 to 5 months). One patient with multiple organ failure died shortly after admission, before the diagnosis was confirmed. None of the patients had undergone gene therapy.
Conclusions: It is important to diagnose ADA SCID as early as possible, before irreversible multi-organ failure occurs. In Poland HSCT are performed according to international immunological societies recommendations, while ERT and GT are less accessible. Implementation of Newborn Screening (NBS) for SCID in Poland could enable recognition of SCID, including ADA-SCID.
Keywords: ERT (enzyme replacement therapy); HSCT = hematopoietic stem cell transplant; SCID - severe combined immunodeficiency; adenosine deaminase (ADA) deficiency; lymphopenia.
Copyright © 2023 Dąbrowska-Leonik, Piątosa, Słomińska, Bohynikova, Bernat-Sitarz, Bernatowska, Wolska-Kuśnierz, Kałwak, Kołtan, Dąbrowska, Goździk, Ussowicz and Pac.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Adenosine Deaminase Deficiency.2006 Oct 3 [updated 2024 Mar 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2006 Oct 3 [updated 2024 Mar 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301656 Free Books & Documents. Review.
-
How We Manage Adenosine Deaminase-Deficient Severe Combined Immune Deficiency (ADA SCID).J Clin Immunol. 2017 May;37(4):351-356. doi: 10.1007/s10875-017-0373-y. Epub 2017 Feb 14. J Clin Immunol. 2017. PMID: 28194615 Review.
-
Consensus approach for the management of severe combined immune deficiency caused by adenosine deaminase deficiency.J Allergy Clin Immunol. 2019 Mar;143(3):852-863. doi: 10.1016/j.jaci.2018.08.024. Epub 2018 Sep 5. J Allergy Clin Immunol. 2019. PMID: 30194989 Free PMC article. Review.
-
ADA Deficiency: Evaluation of the Clinical and Laboratory Features and the Outcome.J Clin Immunol. 2018 May;38(4):484-493. doi: 10.1007/s10875-018-0496-9. Epub 2018 May 9. J Clin Immunol. 2018. PMID: 29744787
-
Adenosine Deaminase (ADA)-Deficient Severe Combined Immune Deficiency (SCID): Molecular Pathogenesis and Clinical Manifestations.J Clin Immunol. 2017 Oct;37(7):626-637. doi: 10.1007/s10875-017-0433-3. Epub 2017 Aug 25. J Clin Immunol. 2017. PMID: 28842866 Review.
Cited by
-
Pathogenesis-driven treatment of primary pulmonary alveolar proteinosis.Eur Respir Rev. 2024 Aug 14;33(173):240064. doi: 10.1183/16000617.0064-2024. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39142709 Free PMC article. Review.
-
Using T-Cell Subsets to Better Characterize Immunoresiliency and Immunodeficiency in Patients with Recurrent Infections.Infect Dis Rep. 2024 Dec 16;16(6):1230-1239. doi: 10.3390/idr16060097. Infect Dis Rep. 2024. PMID: 39728019 Free PMC article.
References
-
- Smolenski RT, Lachno DR, Ledingham SJ, Yacoub MH. Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in hearts for transplantation. J Chromatogr B Biomed Sci Appl (1990) 527:414–20. doi: 10.1016/S0378-4347(00)82125-8 - DOI - PubMed
-
- Piątosa B, Wolska-Kusnierz B, Siewiera K, Grzduk H, Gałkowska E BE, Bernatowska E. Distribution of leukocyte and lymphocyte subsets in peripheral blood. age related normal values for preliminary evaluation of the immune status in polish children. Centr Eur J Immunol (2010) 35:168–75.
-
- Giżewska M, Durda K, Winter T, Ostrowska I, Ołtarzewski M, Klein J, et al. . Newborn screening for SCID and other severe primary immunodeficiency in the polish-German transborder area: Experience from the first 14 months of collaboration. Front Immunol (2020) 11:1948. doi: 10.3389/fimmu.2020.01948 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

