The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives
- PMID: 36685588
- PMCID: PMC9853388
- DOI: 10.3389/fimmu.2022.1089600
The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives
Abstract
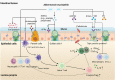
Inflammatory bowel diseases, including Crohn's disease and ulcerative colitis, is a chronic relapsing gastrointestinal inflammatory disease mediated by dysregulated immune responses to resident intestinal microbiota. Current conventional approaches including aminosalicylates, corticosteroids, immunosuppressive agents, and biological therapies are focused on reducing intestinal inflammation besides inducing and maintaining disease remission, and managing complications. However, these therapies are not curative and are associated with various limitations, such as drug resistance, low responsiveness and adverse events. Recent accumulated evidence has revealed the involvement of mucin-degrading bacterium Akkermansia muciniphila (A. muciniphila) in the regulation of host barrier function and immune response, and how reduced intestinal colonisation of probiotic A. muciniphila can contribute to the process and development of inflammatory bowel diseases, suggesting that it may be a potential target and promising strategy for the therapy of inflammatory bowel disease. In this review, we summarise the current knowledge of the role of A. muciniphila in IBD, especially focusing on the related mechanisms, as well as the strategies based on supplementation with A. muciniphila, probiotics and prebiotics, natural diets, drugs, and herbs to promote its colonisation in the gut, and holds promise for A. muciniphila-targeted and -based therapies in the treatment of inflammatory bowel disease.
Keywords: Akkermansia muciniphila; barrier function; gut microbiota; inflammatory bowel disease; mucosal immunity.
Copyright © 2023 Zheng, Han, Yuan, Xing, Zhang, Sun, Liu, Li and Mao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

