Formation of Re-Aggregated Neonatal Porcine Islet Clusters Improves In Vitro Function and Transplantation Outcome
- PMID: 36685665
- PMCID: PMC9846776
- DOI: 10.3389/ti.2022.10697
Formation of Re-Aggregated Neonatal Porcine Islet Clusters Improves In Vitro Function and Transplantation Outcome
Abstract
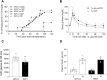
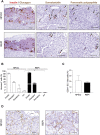
Neonatal porcine islet-like cell clusters (NPICCs) are a promising source for islet cell transplantation. Excellent islet quality is important to achieve a cure for type 1 diabetes. We investigated formation of cell clusters from dispersed NPICCs on microwell cell culture plates, evaluated the composition of re-aggregated porcine islets (REPIs) and compared in vivo function by transplantation into diabetic NOD-SCID IL2rγ-/- (NSG) mice with native NPICCs. Dissociation of NPICCs into single cells and re-aggregation resulted in the formation of uniform REPI clusters. A higher prevalence of normoglycemia was observed in diabetic NSG mice after transplantation with a limited number (n = 1500) of REPIs (85.7%) versus NPICCs (n = 1500) (33.3%) (p < 0.05). Transplanted REPIs and NPICCs displayed a similar architecture of endocrine and endothelial cells. Intraperitoneal glucose tolerance tests revealed an improved beta cell function after transplantation of 1500 REPIs (AUC glucose 0-120 min 6260 ± 305.3) as compared to transplantation of 3000 native NPICCs (AUC glucose 0-120 min 8073 ± 536.2) (p < 0.01). Re-aggregation of single cells from dissociated NPICCs generates cell clusters with excellent functionality and improved in vivo function as compared to native NPICCs.
Keywords: islet transplantation; neonatal islet-like cell clusters; porcine islets; pseudo-islets; re-aggregated cell clusters; xenotransplantation.
Copyright © 2022 Honarpisheh, Lei, Zhang, Pehl, Kemter, Kraetzl, Lange, Wolf, Wolf-van Buerck, Seissler and the VANGUARD consortium.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

