Acoustic Micro-Tapping Optical Coherence Elastography to Quantify Corneal Collagen Cross-Linking: An Ex Vivo Human Study
- PMID: 36685713
- PMCID: PMC9852959
- DOI: 10.1016/j.xops.2022.100257
Acoustic Micro-Tapping Optical Coherence Elastography to Quantify Corneal Collagen Cross-Linking: An Ex Vivo Human Study
Abstract
Purpose: To evaluate changes in the anisotropic elastic properties of ex vivo human cornea treated with ultraviolet cross-linking (CXL) using noncontact acoustic micro-tapping optical coherence elastography (AμT-OCE).
Design: Acoustic micro-tapping OCE was performed on normal and CXL human donor cornea in an ex vivo laboratory study.
Subjects: Normal human donor cornea (n = 22) divided into 4 subgroups. All samples were stored in optisol.
Methods: Elastic properties (in-plane Young's, E, and out-of-plane, G, shear modulus) of normal and ultraviolet CXL-treated human corneas were quantified using noncontact AμT-OCE. A nearly incompressible transverse isotropic model was used to reconstruct moduli from AμT-OCE data. Independently, cornea elastic moduli were also measured with destructive mechanical tests (tensile extensometry and shear rheometry).
Main outcome measures: Corneal elastic moduli (in-plane Young's modulus, E, in-plane, μ, and out-of-plane, G, shear moduli) can be evaluated in both normal and CXL treated tissues, as well as monitored during the CXL procedure using noncontact AμT-OCE.
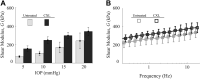
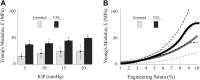
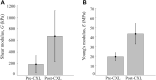
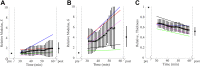
Results: Cross-linking induced a significant increase in both in-plane and out-of-plane elastic moduli in human cornea. The statistical mean in the paired study (presurgery and postsurgery, n = 7) of the in-plane Young's modulus, , increased from 19 MPa to 43 MPa, while the out-of-plane shear modulus, G, increased from 188 kPa to 673 kPa. Mechanical tests in a separate subgroup support CXL-induced cornea moduli changes and generally agree with noncontact AμT-OCE measurements.
Conclusions: The human cornea is a highly anisotropic material where in-plane mechanical properties are very different from those out-of-plane. Noncontact AμT-OCE can measure changes in the anisotropic elastic properties in human cornea as a result of ultraviolet CXL.
Keywords: AμT, acoustic micro-tapping; BSS, balanced saline solution; CXL, cross-linking; Cornea; Cross-linking; Elastic Anisotropy; IOP, intraocular pressure; NITI model; NITI, nearly incompressible transverse isotropy; OCE, optical coherence elastography; Optical Coherence Elastography; RF, riboflavin; UV, ultraviolet.
© 2022 Published by Elsevier Inc. on behalf of American Academy of Ophthalmology.
Figures




References
-
- Meek K.M., Boote C. The organization of collagen in the corneal stroma. Exp Eye Res. 2004;78:503–512. - PubMed
-
- Koudouna E., Winkler M., Mikula E., Juhasz T., et al. Evolution of the vertebrate corneal stroma. Prog Retin Eye Res. 2018;64:65–76. - PubMed
-
- Borcherding M.S., Blacik L.J., Sittig R.A., Bizzell J.W., et al. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res. 1975;21:59–70. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

