Dynamic degradation patterns of porous polycaprolactone/β-tricalcium phosphate composites orchestrate macrophage responses and immunoregulatory bone regeneration
- PMID: 36685731
- PMCID: PMC9832114
- DOI: 10.1016/j.bioactmat.2022.07.032
Dynamic degradation patterns of porous polycaprolactone/β-tricalcium phosphate composites orchestrate macrophage responses and immunoregulatory bone regeneration
Abstract
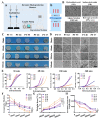
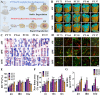
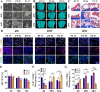
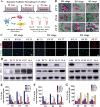
Biodegradable polycaprolactone/β-tricalcium phosphate (PT) composites are desirable candidates for bone tissue engineering applications. A higher β-tricalcium phosphate (TCP) ceramic content improves the mechanical, hydrophilic and osteogenic properties of PT scaffolds in vitro. Using a dynamic degradation reactor, we established a steady in vitro degradation model to investigate the changes in the physio-chemical and biological properties of PT scaffolds during degradation.PT46 and PT37 scaffolds underwent degradation more rapidly than PT scaffolds with lower TCP contents. In vivo studies revealed the rapid degradation of PT (PT46 and PT37) scaffolds disturbed macrophage responses and lead to bone healing failure. Macrophage co-culture assays and a subcutaneous implantation model indicated that the scaffold degradation process dynamically affected macrophage responses, especially polarization. RNA-Seq analysis indicated phagocytosis of the degradation products of PT37 scaffolds induces oxidative stress and inflammatory M1 polarization in macrophages. Overall, this study reveals that the dynamic patterns of biodegradation of degradable bone scaffolds highly orchestrate immune responses and thus determine the success of bone regeneration. Therefore, through evaluation of the biological effects of biomaterials during the entire process of degradation on immune responses and bone regeneration are necessary in order to develop more promising biomaterials for bone regeneration.
Keywords: Bone healing; Dynamic degradation; Macrophage response; Polycaprolactone/β-TCP.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate.Biomaterials. 2014 Oct;35(30):8553-65. doi: 10.1016/j.biomaterials.2014.06.038. Epub 2014 Jul 11. Biomaterials. 2014. PMID: 25017094
-
Degradation and osteogenic potential of a novel poly(lactic acid)/nano-sized β-tricalcium phosphate scaffold.Int J Nanomedicine. 2012;7:5881-8. doi: 10.2147/IJN.S38127. Epub 2012 Nov 28. Int J Nanomedicine. 2012. PMID: 23226019 Free PMC article.
-
In Vitro Mechanical and Biological Properties of 3D Printed Polymer Composite and β-Tricalcium Phosphate Scaffold on Human Dental Pulp Stem Cells.Materials (Basel). 2020 Jul 8;13(14):3057. doi: 10.3390/ma13143057. Materials (Basel). 2020. PMID: 32650530 Free PMC article.
-
Comparative studies on thin polycaprolactone-tricalcium phosphate composite scaffolds and its interaction with mesenchymal stem cells.Biomater Res. 2019 Jan 3;23:1. doi: 10.1186/s40824-018-0153-7. eCollection 2019. Biomater Res. 2019. PMID: 30788137 Free PMC article.
-
The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective.J Funct Biomater. 2019 Mar 22;10(1):16. doi: 10.3390/jfb10010016. J Funct Biomater. 2019. PMID: 30909518 Free PMC article. Review.
Cited by
-
Characterization of Acellular Cartilage Matrix-Sodium Alginate Scaffolds in Various Proportions.Tissue Eng Part C Methods. 2024 Apr;30(4):170-182. doi: 10.1089/ten.TEC.2023.0348. Epub 2024 Mar 20. Tissue Eng Part C Methods. 2024. PMID: 38420649 Free PMC article.
-
Engineering the Immune Response to Biomaterials.Adv Sci (Weinh). 2025 May;12(19):e2414724. doi: 10.1002/advs.202414724. Epub 2025 Apr 15. Adv Sci (Weinh). 2025. PMID: 40232044 Free PMC article. Review.
-
Research progresses on mitochondrial-targeted biomaterials for bone defect repair.Regen Biomater. 2024 Jul 1;11:rbae082. doi: 10.1093/rb/rbae082. eCollection 2024. Regen Biomater. 2024. PMID: 39055307 Free PMC article. Review.
-
The Structural, Thermal and Morphological Characterization of Polylactic Acid/Β-Tricalcium Phosphate (PLA/Β-TCP) Composites upon Immersion in SBF: A Comprehensive Analysis.Polymers (Basel). 2024 Mar 6;16(5):719. doi: 10.3390/polym16050719. Polymers (Basel). 2024. PMID: 38475402 Free PMC article.
-
Histological and Immunohistochemical Characterization of Osteoimmunological Processes in Scaffold-Guided Bone Regeneration in an Ovine Large Segmental Defect Model.Biomedicines. 2023 Oct 13;11(10):2781. doi: 10.3390/biomedicines11102781. Biomedicines. 2023. PMID: 37893154 Free PMC article.
References
-
- Hollinger J.O., Battistone G.C. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin. Orthop. Relat. Res. 1986;207:290–305. - PubMed
LinkOut - more resources
Full Text Sources

