Degeneration of retina-brain components and connections in glaucoma: Disease causation and treatment options for eyesight preservation
- PMID: 36685768
- PMCID: PMC9846481
- DOI: 10.1016/j.crneur.2022.100037
Degeneration of retina-brain components and connections in glaucoma: Disease causation and treatment options for eyesight preservation
Abstract
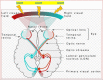
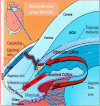
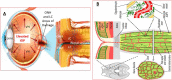
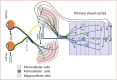
Eyesight is the most important of our sensory systems for optimal daily activities and overall survival. Patients who experience visual impairment due to elevated intraocular pressure (IOP) are often those afflicted with primary open-angle glaucoma (POAG) which slowly robs them of their vision unless treatment is administered soon after diagnosis. The hallmark features of POAG and other forms of glaucoma are damaged optic nerve, retinal ganglion cell (RGC) loss and atrophied RGC axons connecting to various brain regions associated with receipt of visual input from the eyes and eventual decoding and perception of images in the visual cortex. Even though increased IOP is the major risk factor for POAG, the disease is caused by many injurious chemicals and events that progress slowly within all components of the eye-brain visual axis. Lowering of IOP mitigates the damage to some extent with existing drugs, surgical and device implantation therapeutic interventions. However, since multifactorial degenerative processes occur during aging and with glaucomatous optic neuropathy, different forms of neuroprotective, nutraceutical and electroceutical regenerative and revitalizing agents and processes are being considered to combat these eye-brain disorders. These aspects form the basis of this short review article.
Keywords: Axonal injury; Glaucoma; Intraocular pressure; Neurodegeneration; Neuroprotection; Optic nerve; Retina; Retinal ganglion cell.
© 2022 The Author.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Glaucomatous optic neuropathy treatment options: the promise of novel therapeutics, techniques and tools to help preserve vision.Neural Regen Res. 2018 Jul;13(7):1145-1150. doi: 10.4103/1673-5374.235017. Neural Regen Res. 2018. PMID: 30028313 Free PMC article. Review.
-
Upregulation of the endothelin A (ETA) receptor and its association with neurodegeneration in a rodent model of glaucoma.BMC Neurosci. 2017 Mar 1;18(1):27. doi: 10.1186/s12868-017-0346-3. BMC Neurosci. 2017. PMID: 28249604 Free PMC article.
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
-
Glaucoma.2024 Mar 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30855805 Free Books & Documents.
-
[A challenge to primary open-angle glaucoma including normal-pressure. Clinical problems and their scientific solution].Nippon Ganka Gakkai Zasshi. 2012 Mar;116(3):233-67; discussion 268. Nippon Ganka Gakkai Zasshi. 2012. PMID: 22568103 Review. Japanese.
Cited by
-
Stable Gastric Pentadecapeptide BPC 157-Possible Novel Therapy of Glaucoma and Other Ocular Conditions.Pharmaceuticals (Basel). 2023 Jul 24;16(7):1052. doi: 10.3390/ph16071052. Pharmaceuticals (Basel). 2023. PMID: 37513963 Free PMC article. Review.
-
Associations between dementia and exposure to topical glaucoma medications.J Alzheimers Dis. 2025 Feb;103(3):679-686. doi: 10.1177/13872877241305745. Epub 2025 Jan 21. J Alzheimers Dis. 2025. PMID: 39834248
References
-
- Abu-Amero K.K., Morales J., Bosley T.M. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 2006;47(6):2533–2541. - PubMed
Further reading
-
- Aihara M., Lu F., Kawata H., et al. Omidenepag Isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the Phase 3 AYAME Study. Am. J. Ophthalmol. 2020;220:53–63. - PubMed
-
- Alvarado J., Murphy C., Polansky J., Juster R. Age-related changes in trabecular meshwork cellularity. Invest. Ophthalmol. Vis. Sci. 1981;21(5):714–727. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

