Insect biomass density: measurement of seasonal and daily variations using an entomological optical sensor
- PMID: 36685802
- PMCID: PMC9845170
- DOI: 10.1007/s00340-023-07973-5
Insect biomass density: measurement of seasonal and daily variations using an entomological optical sensor
Abstract
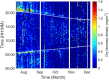
Insects are major actors in Earth's ecosystems and their recent decline in abundance and diversity is alarming. The monitoring of insects is paramount to understand the cause of this decline and guide conservation policies. In this contribution, an infrared laser-based system is used to remotely monitor the biomass density of flying insects in the wild. By measuring the optical extinction caused by insects crossing the 36-m long laser beam, the Entomological Bistatic Optical Sensor System used in this study can evaluate the mass of each specimen. At the field location, between July and December 2021, the instrument made a total of 262,870 observations of insects for which the average dry mass was 17.1 mg and the median 3.4 mg. The daily average mass of flying insects per meter cube of air at the field location has been retrieved throughout the season and ranged between near 0 to 1.2 mg/m3. Thanks to its temporal resolution in the minute range, daily variations of biomass density have been observed as well. These measurements show daily activity patterns changing with the season, as large increases in biomass density were evident around sunset and sunrise during Summer but not during Fall.
© The Author(s) 2023.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures











References
-
- Forister ML, Pelton EM, Black SH. Declines in insect abundance and diversity : we know enough to act now. Conserv. Sci. Pract. 2019 doi: 10.1111/csp2.80. - DOI
-
- Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol. Conserv. 2006;132:279–291. doi: 10.1016/j.biocon.2006.04.020. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
