Comparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophages
- PMID: 36685903
- PMCID: PMC9845402
- DOI: 10.3389/fgene.2022.1051568
Comparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophages
Abstract
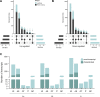
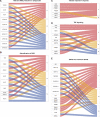
It is well established that infection with Leishmania alters the host cell's transcriptome. Since mammalian cells have multiple mechanisms to control gene expression, different molecules, such as noncoding RNAs, can be involved in this process. MicroRNAs have been extensively studied upon Leishmania infection, but whether long noncoding RNAs (lncRNAs) are also altered in macrophages is still unexplored. We performed RNA-seq from THP-1-derived macrophages infected with Leishmania amazonensis (La), L. braziliensis (Lb), and L. infantum (Li), investigating a previously unappreciated fraction of macrophage transcriptome. We found that more than 24% of the total annotated transcripts and 30% of differentially expressed (DE) RNAs in Leishmania-infected macrophage correspond to lncRNAs. LncRNAs and protein coding RNAs with altered expression are similar among macrophages infected with the Leishmania species. Still, some species-specific alterations could occur due to distinct pathophysiology in which Li infection led to a more significant number of exclusively DE RNAs. The most represented classes among DE lncRNAs were intergenic and antisense lncRNAs. We also found enrichment for immune response-related pathways in the DE protein coding RNAs, as well as putative targets of the lncRNAs. We performed a coexpression analysis to explore potential cis regulation of coding and antisense noncoding transcripts. We identified that antisense lncRNAs are similarly regulated as its neighbor protein coding genes, such as the BAALC/BAALC-AS1, BAALC/BAALC-AS2, HIF1A/HIF1A-AS1, HIF1A/HIF1A-AS3 and IRF1/IRF1-AS1 pairs, which can occur as a species-specific modulation. These findings are a novelty in the field because, to date, no study has focused on analyzing lncRNAs in Leishmania-infected macrophage. Our results suggest that lncRNAs may account for a novel mechanism by which Leishmania can control macrophage function. Further research must validate putative lncRNA targets and provide additional prospects in lncRNA function during Leishmania infection.
Keywords: Leishmania; RNA-seq; THP-1; gene expression; host-parasite interaction; lncRNA; macrophage; transcriptomics.
Copyright © 2023 Fernandes, Gonçalves, Floeter-Winter, Nakaya and Muxel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.Front Immunol. 2022 Mar 15;13:784463. doi: 10.3389/fimmu.2022.784463. eCollection 2022. Front Immunol. 2022. PMID: 35370994 Free PMC article.
-
Long non-coding RNAs: From disease code to drug role.Acta Pharm Sin B. 2021 Feb;11(2):340-354. doi: 10.1016/j.apsb.2020.10.001. Epub 2020 Oct 10. Acta Pharm Sin B. 2021. PMID: 33643816 Free PMC article. Review.
-
Transcriptome analysis of long non-coding RNAs in Mycobacterium avium complex-infected macrophages.Front Immunol. 2024 Apr 22;15:1374437. doi: 10.3389/fimmu.2024.1374437. eCollection 2024. Front Immunol. 2024. PMID: 38711507 Free PMC article.
-
Preliminary RNA-Seq Analysis of Long Non-Coding RNAs Expressed in Human Term Placenta.Int J Mol Sci. 2018 Jun 27;19(7):1894. doi: 10.3390/ijms19071894. Int J Mol Sci. 2018. PMID: 29954144 Free PMC article.
-
Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases.Brain Res Bull. 2013 Aug;97:69-80. doi: 10.1016/j.brainresbull.2013.06.001. Epub 2013 Jun 10. Brain Res Bull. 2013. PMID: 23756188 Review.
Cited by
-
Early Leishmania infectivity depends on miR-372/373/520d family-mediated reprogramming of polyamines metabolism in THP-1-derived macrophages.Sci Rep. 2024 Jan 10;14(1):996. doi: 10.1038/s41598-024-51511-y. Sci Rep. 2024. PMID: 38200138 Free PMC article.
-
Leishmania infantum infection modulates messenger RNA, microRNA and long non-coding RNA expression in human neutrophils in vitro.PLoS Negl Trop Dis. 2024 Jul 19;18(7):e0012318. doi: 10.1371/journal.pntd.0012318. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 39028711 Free PMC article.
-
Integrated analysis of lncRNA and mRNA expression profiles in cutaneous leishmaniasis lesions caused by Leishmania tropica.Front Cell Infect Microbiol. 2024 Nov 21;14:1416925. doi: 10.3389/fcimb.2024.1416925. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39639867 Free PMC article.
-
DNA Methylation in macrophages infected with Leishmania spp. in different culture conditions.Emerg Microbes Infect. 2025 Dec;14(1):2508766. doi: 10.1080/22221751.2025.2508766. Epub 2025 Jun 9. Emerg Microbes Infect. 2025. PMID: 40396886 Free PMC article.
References
-
- Aoki J. I., Muxel S. M., Zampieri R. A., Müller K. E., Nerland A. H., Floeter-Winter L. M. (2019). Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles. Sci. Rep. 9 (1), 19841. 10.1038/s41598-019-56305-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

