Comparative transcriptome analyses of immune responses to LPS in peripheral blood mononuclear cells from the giant panda, human, mouse, and monkey
- PMID: 36685921
- PMCID: PMC9852843
- DOI: 10.3389/fgene.2022.1053655
Comparative transcriptome analyses of immune responses to LPS in peripheral blood mononuclear cells from the giant panda, human, mouse, and monkey
Abstract
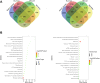
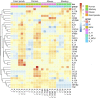
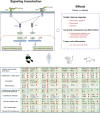
Gram-negative bacteria are major pathogens that can cause illnesses in giant pandas. Lipopolysaccharides (LPS), components of Gram-negative bacteria, can activate immune responses in mammals (i.e., humans and mice) through recognition by toll-like receptors (TLRs). However, the giant pandas' immune response to LPS stimulation and the differences between the giant panda and other mammals are not fully known. In this study, we administrated peripheral blood mononuclear cells (PBMCs) from giant pandas, humans, C57BL/6 mice, and rhesus monkeys by LPS treatment at 6 h followed by RNA sequencing (RNA-seq), respectively, with control of non-stimulation. KEGG analyses of differentially expressed genes (DEGs) pathways indicated that LPS could activate the classic signaling pathway of NF-κB in PBMCs from those four tested species. Thus, similar to the other three species, NF-κB is an LPS-responsive regulator of innate immune responses in giant pandas. Furthermore, the expression patterns of adapter genes, inflammatory cytokine genes, chemokines, interferon genes, cytokine genes related to cell growth and development, costimulatory molecules, Th1/Th2 cytokine genes, Th17 cytokine genes, Th9, and Th22 cytokine genes were compared among giant pandas and three other species. Our data indicated that in addition to the similar expression patterns of certain genes among giant pandas and other species, the unique expression pattern response to LPS in giant pandas was also discovered. Furthermore, Th9, Th17, and Th22 cells might be involved in the response to LPS in giant pandas at this tested time point. This study reveals that LPS-induced immune responses have different sensitivities and response timelines in giant pandas compared with other mammals. This study facilitates further understanding of the role of the TLR signaling pathway and the immune system in giant pandas, which might be helpful for disease prevention and protection.
Keywords: LPS; PBMCs; comparative transcriptome analyses; giant panda; immune responses.
Copyright © 2023 Li, Li, Chen, Yang, Ren, Xu, Wu, Wang, Ling, Shen, Lu, Liu and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Whole genome bisulfite sequencing reveals DNA methylation roles in the adaptive response of wildness training giant pandas to wild environment.Front Genet. 2022 Oct 11;13:995700. doi: 10.3389/fgene.2022.995700. eCollection 2022. Front Genet. 2022. PMID: 36303550 Free PMC article.
-
Blood transcriptome analysis revealed the immune changes and immunological adaptation of wildness training giant pandas.Mol Genet Genomics. 2022 Jan;297(1):227-239. doi: 10.1007/s00438-021-01841-7. Epub 2022 Jan 5. Mol Genet Genomics. 2022. PMID: 34985592
-
Single-cell transcriptomic and chromatin accessibility analyses of dairy cattle peripheral blood mononuclear cells and their responses to lipopolysaccharide.BMC Genomics. 2022 Apr 30;23(1):338. doi: 10.1186/s12864-022-08562-0. BMC Genomics. 2022. PMID: 35501711 Free PMC article.
-
Review on parasites of wild and captive giant pandas (Ailuropoda melanoleuca): Diversity, disease and conservation impact.Int J Parasitol Parasites Wildl. 2020 Jul 28;13:38-45. doi: 10.1016/j.ijppaw.2020.07.007. eCollection 2020 Dec. Int J Parasitol Parasites Wildl. 2020. PMID: 32793415 Free PMC article. Review.
-
Giant pandas are not an evolutionary cul-de-sac: evidence from multidisciplinary research.Mol Biol Evol. 2015 Jan;32(1):4-12. doi: 10.1093/molbev/msu278. Epub 2014 Oct 1. Mol Biol Evol. 2015. PMID: 25274274 Review.
Cited by
-
Pathogen stimulations and immune cells synergistically affect the gene expression profile characteristics of porcine peripheral blood mononuclear cells.BMC Genomics. 2024 Jul 25;25(1):719. doi: 10.1186/s12864-024-10603-9. BMC Genomics. 2024. PMID: 39054472 Free PMC article.
References
-
- Bosteels C., Neyt K., Vanheerswynghels M., van Helden M. J., Sichien D., Debeuf N., et al. (2020). Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 52 (6), 1039–1056. e9. 10.1016/j.immuni.2020.04.005 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

