CRISPR/Cas9-editing of KISS1 to generate pigs with hypogonadotropic hypogonadism as a castration free trait
- PMID: 36685939
- PMCID: PMC9854396
- DOI: 10.3389/fgene.2022.1078991
CRISPR/Cas9-editing of KISS1 to generate pigs with hypogonadotropic hypogonadism as a castration free trait
Abstract
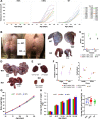
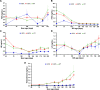
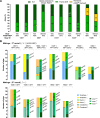
Introduction: Most male pigs are surgically castrated to avoid puberty-derived boar taint and aggressiveness. However, this surgical intervention represents a welfare concern in swine production. Disrupting porcine KISS1 is hypothesized to delay or abolish puberty by inducing variable hypogonadotropism and thus preventing the need for castration. Methods: To test this hypothesis, we generated the first KISS1-edited large animal using CRISPR/Cas9-ribonucleoproteins and single-stranded donor oligonucleotides. The targeted region preceded the sequence encoding a conserved core motif of kisspeptin. Genome editors were intracytoplasmically injected into 684 swine zygotes and transferred to 19 hormonally synchronized surrogate sows. In nine litters, 49 American Yorkshire and 20 Duroc liveborn piglets were naturally farrowed. Results: Thirty-five of these pigs bore KISS1-disruptive alleles ranging in frequency from 5% to 97% and did not phenotypically differ from their wild-type counterparts. In contrast, four KISS1-edited pigs (two boars and two gilts) with disruptive allele frequencies of 96% and 100% demonstrated full hypogonadotropism, infantile reproductive tracts, and failed to reach sexual maturity. Change in body weight during development was unaffected by editing KISS1. Founder pigs partially carrying KISS1-disruptive alleles were bred resulting in a total of 53 KISS1 +/+, 60 KISS1 +/-, and 34 KISS1 -/- F1 liveborn piglets, confirming germline transmission. Discussion: Results demonstrate that a high proportion of KISS1 alleles in pigs must be disrupted before variation in gonadotropin secretion is observed, suggesting that even a small amount of kisspeptin ligand is sufficient to confer proper sexual development and puberty in pigs. Follow-on studies will evaluate fertility restoration in KISS1 KO breeding stock to fully realize the potential of KISS1 gene edits to eliminate the need for surgical castration.
Keywords: animal welfare; boar taint; embryo editing; homology-directed repair; kisspeptin; knockout; pig puberty.
Copyright © 2023 Flórez, Martins, Solin, Bostrom, Rodríguez-Villamil, Ongaratto, Larson, Ganbaatar, Coutts, Kern, Murphy, Kim, Carlson, Huisman, Sonstegard and Lents.
Conflict of interest statement
The authors JF, KM, JB, PR-V, FO, SL, E-SK, and TS are employed by Acceligen Inc., a wholly owned subsidiary of Recombinetics Inc.; SS, UG, AC, and DC are employed by Recombinetics Inc. AH is an employee of Hendrix Genetics. These companies contributed supplemental funding for this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Development of KISS1 knockout pigs is characterized by hypogonadotropic hypogonadism, normal growth, and reduced skatole†.Biol Reprod. 2024 Nov 11;111(5):1082-1096. doi: 10.1093/biolre/ioae140. Biol Reprod. 2024. PMID: 39375014
-
The effect of the MC4R gene on boar taint compounds, sexual maturity and behaviour in growing-finishing boars and gilts.Animal. 2015 Oct;9(10):1688-97. doi: 10.1017/S1751731115001135. Epub 2015 Jul 9. Animal. 2015. PMID: 26155873
-
Neonatal Kisspeptin is Steroid-Independently Required for Defeminisation and Peripubertal Kisspeptin-Induced Testosterone is Required for Masculinisation of the Brain: A Behavioural Study Using Kiss1 Knockout Rats.J Neuroendocrinol. 2016 Oct;28(10). doi: 10.1111/jne.12409. J Neuroendocrinol. 2016. PMID: 27344056
-
The roles of kisspeptin in the mechanism underlying reproductive functions in mammals.J Reprod Dev. 2018 Dec 14;64(6):469-476. doi: 10.1262/jrd.2018-110. Epub 2018 Oct 8. J Reprod Dev. 2018. PMID: 30298825 Free PMC article. Review.
-
Kisspeptin/GPR54 system as potential target for endocrine disruption of reproductive development and function.Int J Androl. 2010 Apr;33(2):360-8. doi: 10.1111/j.1365-2605.2009.01012.x. Epub 2009 Nov 10. Int J Androl. 2010. PMID: 19906185 Review.
Cited by
-
Horizon scanning of potential environmental applications of terrestrial animals, fish, algae and microorganisms produced by genetic modification, including the use of new genomic techniques.Front Genome Ed. 2024 Jun 13;6:1376927. doi: 10.3389/fgeed.2024.1376927. eCollection 2024. Front Genome Ed. 2024. PMID: 38938511 Free PMC article.
-
Male animal sterilization: history, current practices, and potential methods for replacing castration.Front Vet Sci. 2024 Jul 3;11:1409386. doi: 10.3389/fvets.2024.1409386. eCollection 2024. Front Vet Sci. 2024. PMID: 39027909 Free PMC article. Review.
-
A bibliometric mapping of advancements and trends in genome editing in pigs.Trop Anim Health Prod. 2025 Apr 30;57(4):201. doi: 10.1007/s11250-025-04432-5. Trop Anim Health Prod. 2025. PMID: 40304841 Review.
-
Gene editing in livestock: innovations and applications.Anim Reprod. 2024 Sep 23;21(3):e20240054. doi: 10.1590/1984-3143-AR2024-0054. eCollection 2024. Anim Reprod. 2024. PMID: 39372257 Free PMC article.
References
-
- American Veterinary Medical Association (2020). AVMA guidelines for the euthanasia of animals: 2020 edition. Available at: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasi... (Accessed February 24, 2022).
-
- Backus G., Higuera M., Juul N., Nalon E., de Briyne N. (2018). Second progress report 2015 – 2017 on the European declaration on alternatives to surgical castration of pigs. Brussels: Available at: https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress... (Accessed December 12, 2022).
LinkOut - more resources
Full Text Sources
Research Materials

