Versatile electrical stimulator for cardiac tissue engineering-Investigation of charge-balanced monophasic and biphasic electrical stimulations
- PMID: 36686253
- PMCID: PMC9846083
- DOI: 10.3389/fbioe.2022.1031183
Versatile electrical stimulator for cardiac tissue engineering-Investigation of charge-balanced monophasic and biphasic electrical stimulations
Abstract
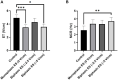
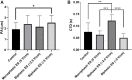
The application of biomimetic physical stimuli replicating the in vivo dynamic microenvironment is crucial for the in vitro development of functional cardiac tissues. In particular, pulsed electrical stimulation (ES) has been shown to improve the functional properties of in vitro cultured cardiomyocytes. However, commercially available electrical stimulators are expensive and cumbersome devices while customized solutions often allow limited parameter tunability, constraining the investigation of different ES protocols. The goal of this study was to develop a versatile compact electrical stimulator (ELETTRA) for biomimetic cardiac tissue engineering approaches, designed for delivering controlled parallelizable ES at a competitive cost. ELETTRA is based on an open-source micro-controller running custom software and is combinable with different cell/tissue culture set-ups, allowing simultaneously testing different ES patterns on multiple samples. In particular, customized culture chambers were appositely designed and manufactured for investigating the influence of monophasic and biphasic pulsed ES on cardiac cell monolayers. Finite element analysis was performed for characterizing the spatial distributions of the electrical field and the current density within the culture chamber. Performance tests confirmed the accuracy, compliance, and reliability of the ES parameters delivered by ELETTRA. Biological tests were performed on neonatal rat cardiac cells, electrically stimulated for 4 days, by comparing, for the first time, the monophasic waveform (electric field = 5 V/cm) to biphasic waveforms by matching either the absolute value of the electric field variation (biphasic ES at ±2.5 V/cm) or the total delivered charge (biphasic ES at ±5 V/cm). Findings suggested that monophasic ES at 5 V/cm and, particularly, charge-balanced biphasic ES at ±5 V/cm were effective in enhancing electrical functionality of stimulated cardiac cells and in promoting synchronous contraction.
Keywords: biomimetic approach; biphasic waveform; cardiac tissue engineering; charge balance; electrical stimulation; in vitro models; monophasic waveform; parallel investigation.
Copyright © 2023 Gabetti, Sileo, Montrone, Putame, Audenino, Marsano and Massai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Baumgartner S., Halbach M., Krausgrill B., Maass M., Srinivasan S. P., Sahito R. G. A., et al. (2015). Electrophysiological and morphological maturation of murine fetal cardiomyocytes during electrical stimulation in vitro . J. Cardiovasc. Pharmacol. Ther. 20, 104–112. 10.1177/1074248414536273 - DOI - PubMed
-
- Béland J., Duverger J. E., Petitjean E., Maguy A., Ledoux J., Comtois P. (2020). Development of an open hardware bioreactor for optimized cardiac cell culture integrating programmable mechanical and electrical stimulations. AIP Adv. 10, 035133. 10.1063/1.5144922 - DOI
LinkOut - more resources
Full Text Sources

