Gelatin-grafted tubular asymmetric scaffolds promote ureteral regeneration via activation of the integrin/Erk signaling pathway
- PMID: 36686259
- PMCID: PMC9849368
- DOI: 10.3389/fbioe.2022.1092543
Gelatin-grafted tubular asymmetric scaffolds promote ureteral regeneration via activation of the integrin/Erk signaling pathway
Abstract
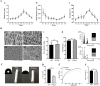
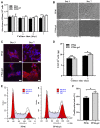
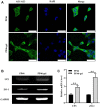
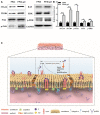
Introduction: The repair of a diseased ureter is an urgent clinical issue that needs to be solved. A tissue-engineered scaffold for ureteral replacement is currently insufficient due to its incompetent bioactivity, especially in long-segment abnormalities. The primary reason is the failure of urothelialization on scaffolds. Methods: In this work, we investigated the ability of gelatin-grafted tubular scaffold in ureteral repairment and its related biological mechanism. We designed various porous asymmetric poly (L-lactic acid) (PLLA)/poly (L-lactide-co-e-caprolactone) (PLCL) tubes with a thermally induced phase separation (TIPS) method via a change in the ratio of solvents (named PP). To regulate the phenotype of urothelial cells and ureteral reconstruction, gelatin was grafted onto the tubular scaffold using ammonolysis and glutaraldehyde crosslinking (named PP-gel). The in vitro and in vivo experiments were performed to test the biological function and the mechanism of the scaffolds. Results and Discussion: The hydrophilicity of the scaffold significantly increased after gelatin grafting, which promoted the adhesion and proliferation of urothelial cells. Through subcutaneous implantation in rats, PP-gel scaffolds demonstrated good biocompatibility. The in vivo replacement showed that PP-gel could improve urothelium regeneration and maintain renal function after the ureter was replaced with an ∼4 cm-long PP-gel tube using New Zealand rabbits as the experimental animals. The related biologic mechanism of ureteral reconstruction was detected in detail. The gelatin-grafted scaffold upgraded the integrin α6/β4 on the urothelial cell membrane, which phosphorylates the focal adhesion kinase (FAK) and enhances urothelialization via the MAPK/Erk signaling pathway. Conclusion: All these results confirmed that the PP46-gel scaffold is a promising candidate for the constitution of an engineered ureter and to repair long-segment ureteral defects.
Keywords: MAPK/ERK pathway; gelatin; tissue engineering; ureter; urothelialization.
Copyright © 2023 Song, Fang, Mao, Ye, Yan, Ma, Shi, Hu, Zhu and Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Al-Maawi S., Rother S., Halfter N., Fiebig K. M., Moritz J., Moeller S., et al. (2022). Covalent linkage of sulfated hyaluronan to the collagen scaffold Mucograft® enhances scaffold stability and reduces proinflammatory macrophage activation in vivo . Bioact. Mater 8, 420–434. 10.1016/j.bioactmat.2021.06.008 - DOI - PMC - PubMed
-
- Dalton G. D., Carney S. T., Marshburn J. D., Norford D. C., Howlett A. C. (2020). CB1 cannabinoid receptors stimulate gβγ-GRK2-mediated FAK phosphorylation at tyrosine 925 to regulate ERK activation involving neuronal focal adhesions. Front. Cell Neurosci. 14, 176. 10.3389/fncel.2020.00176 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

