Better antimicrobial resistance data analysis and reporting in less time
- PMID: 36686270
- PMCID: PMC9847555
- DOI: 10.1093/jacamr/dlac143
Better antimicrobial resistance data analysis and reporting in less time
Abstract
Objectives: Insights about local antimicrobial resistance (AMR) levels and epidemiology are essential to guide decision-making processes in antimicrobial use. However, dedicated tools for reliable and reproducible AMR data analysis and reporting are often lacking. We aimed to compare traditional data analysis and reporting versus a new approach for reliable and reproducible AMR data analysis in a clinical setting.
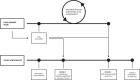
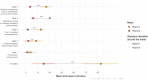
Methods: Ten professionals who routinely work with AMR data were provided with blood culture test results including antimicrobial susceptibility results. Participants were asked to perform a detailed AMR data analysis in a two-round process: first using their software of choice and next using our newly developed software tool. Accuracy of the results and time spent were compared between both rounds. Finally, participants rated the usability using the System Usability Scale (SUS).
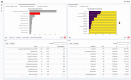
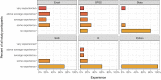
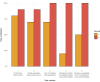
Results: The mean time spent on creating the AMR report reduced from 93.7 to 22.4 min (P < 0.001). Average task completion per round changed from 56% to 96% (P < 0.05). The proportion of correct answers in the available results increased from 37.9% in the first to 97.9% in the second round (P < 0.001). Usability of the new tools was rated with a median of 83.8 (out of 100) on the SUS.
Conclusions: This study demonstrated the significant improvement in efficiency and accuracy in standard AMR data analysis and reporting workflows through open-source software. Integrating these tools in clinical settings can democratize the access to fast and reliable insights about local microbial epidemiology and associated AMR levels. Thereby, our approach can support evidence-based decision-making processes in the use of antimicrobials.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
-
- O’Neill J. Review on Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. https://wellcomecollection.org/works/rdpck35v/items.
-
- OECD . Stemming the superbug tide. 2018. 10.1787/9789264307599-en - DOI
LinkOut - more resources
Full Text Sources
