Lutein nanodisks protect human retinal pigment epithelial cells from UV light-induced damage
- PMID: 36686279
- PMCID: PMC9851610
- DOI: 10.3389/fnano.2022.955022
Lutein nanodisks protect human retinal pigment epithelial cells from UV light-induced damage
Abstract
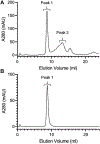
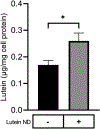
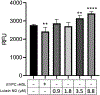
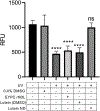
The hydrophobic carotenoid, lutein, was conferred with aqueous solubility upon formulation into reconstituted discoidal high density lipoprotein particles, termed lutein nanodisks (ND). When formulated with phosphatidylcholine (PC), apolipoprotein (apo) A-I and lutein (formulation ratio = 5 mg PC/2 mg apoA-I/1 mg lutein), lutein solubilization efficiency in phosphate buffered saline (PBS) was ~90%. The UV/Vis absorbance maxima for lutein ND in PBS were red shifted by 6-13 nm versus the corresponding lutein absorbance maxima in ethanol. FPLC gel filtration chromatography gave rise to a single major absorbance peak in the size range of ND. Incubation of cultured ARPE-19 cells with lutein ND resulted in lutein uptake, as determined by HPLC analysis of cell extracts. Compared to control incubations, ARPE-19 cells incubated with lutein ND were protected from UV light-induced loss of cell viability. Consistent with this, reactive oxygen species generation, induced by exposure to UV irradiation, was lower in lutein-enriched cells than in control cells. Thus, uptake of ND-associated lutein protects ARPE-19 cells from UV light-induced damage. Taken together, the data indicate ND provide an aqueous lutein delivery vehicle for biotechnological or therapeutic applications.
Keywords: UV irradiation; lutein; macular degeneration; nanodisc; retinal pigment epithelial cells.
Conflict of interest statement
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


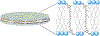
References
-
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch. Ophthalmol 119, 1417–1436. doi:10.1001/archopht.119.10.1417 - DOI - PMC - PubMed
-
- Babizhayev MA (2011). Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract. Cell. biochem. Funct 29, 183–206. doi:10.1002/cbf.1737 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
