Ru(II) CONTAINING PHOTOSENSITIZERS FOR PHOTODYNAMIC THERAPY: A CRITIQUE ON REPORTING AND AN ATTEMPT TO COMPARE EFFICACY
- PMID: 36686369
- PMCID: PMC9850455
- DOI: 10.1016/j.ccr.2022.214712
Ru(II) CONTAINING PHOTOSENSITIZERS FOR PHOTODYNAMIC THERAPY: A CRITIQUE ON REPORTING AND AN ATTEMPT TO COMPARE EFFICACY
Abstract
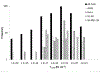
Ruthenium(II)-based coordination complexes have emerged as photosensitizers (PSs) for photodynamic therapy (PDT) in oncology as well as antimicrobial indications and have great potential. Their modular architectures that integrate multiple ligands can be exploited to tune cellular uptake and subcellular targeting, solubility, light absorption, and other photophysical properties. A wide range of Ru(II) containing compounds have been reported as PSs for PDT or as photochemotherapy (PCT) agents. Many studies employ a common scaffold that is subject to systematic variation in one or two ligands to elucidate the impact of these modifications on the photophysical and photobiological performance. Studies that probe the excited state energies and dynamics within these molecules are of fundamental interest and are used to design next-generation systems. However, a comparison of the PDT efficacy between Ru(II) containing PSs and 1st or 2nd generation PSs, already in clinical use or preclinical/clinical studies, is rare. Even comparisons between Ru(II) containing molecular structures are difficult, given the wide range of excitation wavelengths, power densities, and cell lines utilized. Despite this gap, PDT dose metrics quantifying a PS's efficacy are available to perform qualitative comparisons. Such models are independent of excitation wavelength and are based on common outcome parameters, such as the photon density absorbed by the Ru(II) compound to cause 50% cell kill (LD50) based on the previously established threshold model. In this focused photophysical review, we identified all published studies on Ru(II) containing PSs since 2005 that reported the required photophysical, light treatment, and in vitro outcome data to permit the application of the Photodynamic Threshold Model to quantify their potential efficacy. The resulting LD50 values range from less than 1013 to above 1020 [hν cm-3], indicating a wide range in PDT efficacy and required optical energy density for ultimate clinical translation.
Figures


References
-
- Monro S, Colón KL, Yin H, Roque J, Konda P, Gujar S, Thummel RP, Lilge L, Cameron CG, McFarland SA, Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433, Chemical Reviews. 119 (2019) 797–828. 10.1021/acs.chemrev.8b00211. - DOI - PMC - PubMed
-
- Lilge L, Roufaiel M, Lazic S, Kaspler P, Munegowda MA, Nitz M, Bassan J, Mandel A, Evaluation of a Ruthenium coordination complex as photosensitizer for PDT of bladder cancer: Cellular response, tissue selectivity and in vivo response, Translational Biophotonics. 2 (2020). 10.1002/tbio.201900032. - DOI
-
- Clarke MJ, Ruthenium metallopharmaceuticals, Coordination Chemistry Reviews. 236 (2003) 209–233. 10.1016/S0010-8545(02)00312-0. - DOI
-
- Cassells I, Stringer T, Hutton AT, Prince S, Smith GS, Impact of various lipophilic substituents on ruthenium(II), rhodium(III) and iridium(III) salicylaldimine-based complexes: synthesis, in vitro cytotoxicity studies and DNA interactions, JBIC Journal of Biological Inorganic Chemistry. 23 (2018). 10.1007/s00775-018-1567-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
