3D blood-brain barrier-organoids as a model for Lyme neuroborreliosis highlighting genospecies dependent organotropism
- PMID: 36686395
- PMCID: PMC9851883
- DOI: 10.1016/j.isci.2022.105838
3D blood-brain barrier-organoids as a model for Lyme neuroborreliosis highlighting genospecies dependent organotropism
Abstract
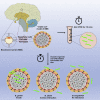
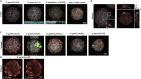
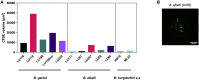
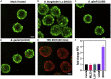
Lyme neuroborreliosis (LNB), a tick-borne infection caused by spirochetes within the Borrelia burgdorferi sensu lato (s.L.) complex, is among the most prevalent bacterial central nervous system (CNS) infections in Europe and the US. Here we have screened a panel of low-passage B. burgdorferi s.l. isolates using a novel, human-derived 3D blood-brain barrier (BBB)-organoid model. We show that human-derived BBB-organoids support the entry of Borrelia spirochetes, leading to swelling of the organoids and a loss of their structural integrity. The use of the BBB-organoid model highlights the organotropism between B. burgdorferi s.l. genospecies and their ability to cross the BBB contributing to CNS infection.
Keywords: Cell biology; Medical Microbiology; Neuroscience.
© 2022.
Conflict of interest statement
Disclosures: Outside of the present work: A.M.L. reports speaker’s honorarium/travel grants and advisory board activity from Gilead, GSK, and Pfizer. The other authors declare no competing interests exist. P.E.L. has been an external scientific expert to Valneva Austria GmbH and Pfizer Inc. and received speaker’s honorarium and travel grants.
Figures