Enantioselective Inter- and Intramolecular Sulfenofunctionalization of Unactivated Cyclic and (Z)-Alkenes
- PMID: 36686398
- PMCID: PMC9851372
- DOI: 10.1021/acscatal.2c01232
Enantioselective Inter- and Intramolecular Sulfenofunctionalization of Unactivated Cyclic and (Z)-Alkenes
Abstract
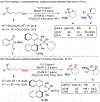
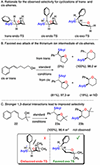
A method for the enantioselective, Lewis base-catalyzed sulfenofunctionalization of cyclic and (Z)-alkenes is reported. The intermediate thiiranium ion generated in the presence of a selenophosphoramide catalyst is intercepted by a variety of nucleophiles. A diverse array of inter- and intramolecular functionalizations proceed in high yield and good to high enantioselectivity (86:14-98:2 er). Prior experimental and computational studies indicated such enantiotopic face discrimination to be poor; however, the results disclosed herein remediate the previous findings. Control experiments were performed to investigate the different behavior of (Z)-alkenes and their more established (E)-counterparts.
Keywords: Lewis base; organocatalysis; sulfenium; sulfenofunctionalization; thiiranium.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
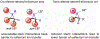
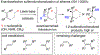

References
-
-
For a review on organocatalytic, enantioselective epoxidation of alkenes see:
- Zhu Y; Wang Q; Cornwall RG; Shi Y Organocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic Applications. Chem. Rev 2014, 114, 8199–8256. - PubMed
-
For relevant book chapters on epoxidation see:
- Katsuki T; Martin VS Asymmetric Epoxidation of Allylic Alcohols: The Katuski-Sharpless Epoxidation Reaction. Org. React 1996, 48, 1–208, Chapter 1.
- Katsuki T Catalytic Asymmetric Synthesis, 2nd ed.; Ojima I, Ed.; Wiley-VCH, 2000, Chapter 6B.
-
-
-
For relevant book chapters on aziridination see:
- Muchalski H; Johnston JN Science of Synthesis, de Vries JG, Ed.; Georg Thieme Velag KG, 2011; Vol. 1, pp 155–184.
- Mossner C; Bolm C Transition Metals for Organic Synthesis, 2nd ed.; Beller M; Bolm C, Eds.; Wiley-WCH Verlag: Weinheim, 2004; pp 389–402.
-
-
- Denmark SE; Collins WR Lewis Base Activation of Lewis Acids: Development of a Lewis Base Catalyzed Selenolactonization. Org. Lett 2007, 9, 3801–3804. - PubMed
- Denmark SE; Kalyani D; Collins WR Preparative and Mechanistic Studies toward the Rational Development of Catalytic, Enantioselective, Selenoetherification Reactions. J. Am. Chem. Soc 2010, 132, 15752–15765. - PMC - PubMed
- Wei Q; Wang Y-Y; Du Y-L; Gong L-Z Organocatalytic Asymmetric Selenofunctionalization of Tryptamine for the Synthesis of Hexahydropyrrolo[2,3-b]indole Derivatives. Beilstein J. Org. Chem 2013, 9, 1559–1564. - PMC - PubMed
- Zhang H; Lin S; Jacobsen EN Enantioselective Selenocyclization via Dynamic Kinetic Resolution of Seleniranium Ions by Hydrogen-Bond Donor Catalysts. J. Am. Chem. Soc 2014, 136, 16485–16488. - PMC - PubMed
- Niu W; Yeung Y-Y Catalytic and Highly Enantioselective Selenolactonization. Org. Lett 2015, 17, 1660–1663. - PubMed
- See JY; Yang H; Zhao Y; Wong MW; Ke Z; Yeung Y-Y Desymmetrizing Enantio- and Diastereoselective Selenoetherification through Supramolecular Catalysis. ACS Catal. 2018, 8, 850–858.
Grants and funding
LinkOut - more resources
Full Text Sources
