A novel coumarin-triazole-thiophene hybrid: synthesis, characterization, ADMET prediction, molecular docking and molecular dynamics studies with a series of SARS-CoV-2 proteins
- PMID: 36686402
- PMCID: PMC9845830
- DOI: 10.1007/s12039-022-02127-0
A novel coumarin-triazole-thiophene hybrid: synthesis, characterization, ADMET prediction, molecular docking and molecular dynamics studies with a series of SARS-CoV-2 proteins
Abstract
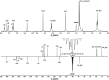
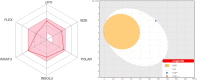
Synthesis, characterization and theoretical studies of a novel coumarin-triazole-thiophene hybrid 4-(((4-ethyl-5-(thiophen-2-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)-6,7-dimethyl-2H-chromen-2-one (1), which was fabricated from 4-ethyl-5-(thiophen-2-yl)-4H-1,2,4-triazole-3-thiol and 4-(chloromethyl)-6,7-dimethyl-2H-chromen-2-one, are reported. The resulting compound was characterized by microanalysis, IR, 1H, and 13C APT NMR spectroscopy. The DFT calculations examined the structure and electronic properties of 1 in gas phase. Its reactivity descriptors and molecular electrostatic potential revealed the reactivity and the reactive centers of 1. ADMET properties of 1 were evaluated using the respective online tools. It was established that 1 exhibit positive gastrointestinal absorption properties and negative human blood-brain barrier penetration. The Toxicity Model Report revealed that 1 belongs to toxicity class 4. Molecular docking was additionally applied to study the interaction of 1 with some SARS-CoV-2 proteins. It was established that the title compound is active against all the applied proteins with the most efficient interaction with Papain-like protease (PLpro). The interaction of 1 with the applied proteins was also studied using molecular dynamics simulations.
Graphical abstract: A novel coumarin-triazole-thiophene hybrid 4-(((4-ethyl-5-(thiophen-2-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)-6,7-dimethyl-2H-chromen-2-one (1) is reported. The structure and electronic properties of 1 were examined by the DFT calculations. ADMET properties of 1 were also evaluated. Molecular docking and molecular dynamics simulations were applied to study interactions of 1 with a series of the SARS-CoV-2 proteins.
Supplementary information: The online version contains supplementary material available at 10.1007/s12039-022-02127-0.
Keywords: Computational study; Coumarin; DFT; Synthesis; Thiophene; Triazole.
© Indian Academy of Sciences 2023.
Figures







References
-
- Thematic issue “Heterocyclic Compounds in Medicinal Chemistry” 2020 Chem. Heterocycl. Comp.56 625
LinkOut - more resources
Full Text Sources
Miscellaneous
