Sequential algorithm to stratify liver fibrosis risk in overweight/obese metabolic dysfunction-associated fatty liver disease
- PMID: 36686469
- PMCID: PMC9853017
- DOI: 10.3389/fendo.2022.1056562
Sequential algorithm to stratify liver fibrosis risk in overweight/obese metabolic dysfunction-associated fatty liver disease
Abstract
Background: Non-diabetic overweight/obese metabolic dysfunction-associated fatty liver disease (MAFLD) represents the largest subgroup with heterogeneous liver fibrosis risk. Metabolic dysfunction promotes liver fibrosis. Here, we investigated whether incorporating additional metabolic risk factors into clinical evaluation improved liver fibrosis risk stratification among individuals with non-diabetic overweight/obese MAFLD.
Materials and methods: Comprehensive metabolic evaluation including 75-gram oral glucose tolerance test was performed in over 1000 participants from the New Hong Kong Cardiovascular Risk Factor Prevalence Study (HK-NCRISPS), a contemporary population-based study of HK Chinese. Hepatic steatosis and fibrosis were evaluated based on controlled attenuation parameter and liver stiffness (LS) measured using vibration-controlled transient elastography, respectively. Clinically significant liver fibrosis was defined as LS ≥8.0 kPa. Our findings were validated in an independent pooled cohort comprising individuals with obesity and/or polycystic ovarian syndrome.
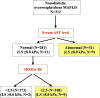
Results: Of the 1020 recruited community-dwelling individuals, 312 (30.6%) had non-diabetic overweight/obese MAFLD. Among them, 6.4% had LS ≥8.0 kPa. In multivariable stepwise logistic regression analysis, abnormal serum aspartate aminotransferase (AST) (OR 7.95, p<0.001) and homeostasis model assessment of insulin resistance (HOMA-IR) ≥2.5 (OR 5.01, p=0.008) were independently associated with LS ≥8.0 kPa, in a model also consisting of other metabolic risk factors including central adiposity, hypertension, dyslipidaemia and prediabetes. A sequential screening algorithm using abnormal AST, followed by elevated HOMA-IR, was developed to identify individuals with LS ≥8.0 kPa, and externally validated with satisfactory sensitivity (>80%) and negative predictive value (>90%).
Conclusion: A sequential algorithm incorporating AST and HOMA-IR levels improves fibrosis risk stratification among non-diabetic overweight/obese MAFLD individuals.
Keywords: MAFLD (metabolic associated fatty liver disease); fatty liver disease; obesity; overweight; population based study.
Copyright © 2023 Lee, Lui, Li, Yuen, Fong, Leung, Chu, Mak, Lam, Woo, Woo, Xu, Tse, Tan, Cheung, Yuen and Lam.
Conflict of interest statement
C-HL received speaker’s fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Sanofi Aventis. M-FY reports grant/research support from AbbVie, Assembly Biosciences, Arrowhead Pharmaceuticals, Bristol Myers Squibb, Fujirebio Incorporation, Gilead Sciences, Immuncore, Merck Sharp and Dohme, Springbank Pharmaceuticals, Sysmex Corporation and Roche, consultancy for AbbVie, Aligos Therapeutics, AiCuris, Antios Therapeutics, Arbutus Biopharma, Arrowhead Pharmaceuticals, Assembly Biosciences, Bristol Myers Squibb, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio Incorporation, GSK, Gilead Sciences, Immunocore, Janssen, Merck Sharp and Dohme, Roche, Springbank Pharmaceuticals, Silverback Therapeutics, Sysmex Corporation and Vir Biotechnology, and lecture fees from AbbVie, Dicerna Pharmaceuticals, Fujirebio Incorporation, Gilead Sciences, Merck Sharp and Dohme, Roche and Sysmex Corporation. KL is an advisory board member of Merck Sharp and Dohme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
Similar articles
-
Simple proxies of insulin resistance identify obese metabolic dysfunction-associated fatty liver disease subjects with advanced liver disease.Diabetes Metab Res Rev. 2024 Feb;40(2):e3736. doi: 10.1002/dmrr.3736. Epub 2023 Oct 15. Diabetes Metab Res Rev. 2024. PMID: 37839068
-
Evaluating future risk of NAFLD in adolescents: a prediction and decision curve analysis.BMC Gastroenterol. 2022 Jun 30;22(1):323. doi: 10.1186/s12876-022-02401-y. BMC Gastroenterol. 2022. PMID: 35773644 Free PMC article.
-
Prevalence and Risk Factors of Metabolic Dysfunction-Associated Fatty Liver Disease with Renal Insufficiency in Overweight/Obese Adults.Obes Facts. 2023;16(6):548-558. doi: 10.1159/000533626. Epub 2023 Aug 28. Obes Facts. 2023. PMID: 37640023 Free PMC article.
-
Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults.Clin Gastroenterol Hepatol. 2022 Mar;20(3):e573-e582. doi: 10.1016/j.cgh.2021.02.030. Epub 2021 Feb 20. Clin Gastroenterol Hepatol. 2022. PMID: 33618024
-
Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2023 Jan;8(1):20-30. doi: 10.1016/S2468-1253(22)00317-X. Epub 2022 Nov 16. Lancet Gastroenterol Hepatol. 2023. PMID: 36400097
References
-
- Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. . Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology (2020) 158:1611–25.e12. doi: 10.1053/j.gastro.2020.01.043 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

