Growth hormone stimulates lipolysis in mice but not in adipose tissue or adipocyte culture
- PMID: 36686475
- PMCID: PMC9846043
- DOI: 10.3389/fendo.2022.1028191
Growth hormone stimulates lipolysis in mice but not in adipose tissue or adipocyte culture
Abstract
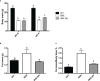
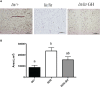
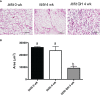
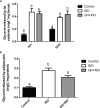
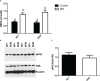
The inhibitory effect of growth hormone (GH) on adipose tissue growth and the stimulatory effect of GH on lipolysis are well known, but the mechanisms underlying these effects are not completely understood. In this study, we revisited the effects of GH on adipose tissue growth and lipolysis in the lit/lit mouse model. The lit/lit mice are GH deficient because of a mutation in the GH releasing hormone receptor gene. We found that the lit/lit mice had more subcutaneous fat and larger adipocytes than their heterozygous lit/+ littermates and that these differences were partially reversed by 4-week GH injection. We also found that GH injection to the lit/lit mice caused the mature adipose tissue and adipocytes to reduce in size. These results demonstrate that GH inhibits adipose tissue growth at least in part by stimulating lipolysis. To determine the mechanism by which GH stimulates lipolysis, we cultured adipose tissue explants and adipocytes derived from lit/lit mice with GH and/or isoproterenol, an agonist of the beta-adrenergic receptors. These experiments showed that whereas isoproterenol, expectedly, stimulated potent lipolysis, GH, surprisingly, had no effect on basal lipolysis or isoproterenol-induced lipolysis in adipose tissue explants or adipocytes. We also found that both isoproterenol-induced lipolysis and phosphorylation of hormone-sensitive lipase were not different between lit/lit and lit/+ mice. Taken together, these results support the conclusion that GH has lipolytic effect in mice but argue against the notion that GH stimulates lipolysis by directly acting on adipocytes or by enhancing β-adrenergic receptors-mediated lipolysis.
Keywords: adipocytes; adipose tissue; adrenergic; growth hormone; lipolysis.
Copyright © 2023 Zhao and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

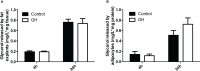
Similar articles
-
Association of growth hormone deficiency with an increased number of preadipocytes in subcutaneous fat.Front Endocrinol (Lausanne). 2023 May 26;14:1199589. doi: 10.3389/fendo.2023.1199589. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37305046 Free PMC article.
-
Growth hormone treatment of hypophysectomized rats increases catecholamine-induced lipolysis and the number of beta-adrenergic receptors in adipocytes: no differences in the effects of growth hormone on different fat depots.Obes Res. 1996 Sep;4(5):471-8. doi: 10.1002/j.1550-8528.1996.tb00256.x. Obes Res. 1996. PMID: 8885212
-
The lipolytic effects of thyrotropin and isoprenaline are not affected by growth hormone in infant adipocytes.Horm Metab Res. 1997 Apr;29(4):164-7. doi: 10.1055/s-2007-979013. Horm Metab Res. 1997. PMID: 9178024
-
The effects of growth hormone on adipose tissue: old observations, new mechanisms.Nat Rev Endocrinol. 2020 Mar;16(3):135-146. doi: 10.1038/s41574-019-0280-9. Epub 2019 Nov 28. Nat Rev Endocrinol. 2020. PMID: 31780780 Free PMC article. Review.
-
Effects of growth hormone and prolactin on adipose tissue development and function.Pituitary. 2003 Sep;6(2):97-102. doi: 10.1023/b:pitu.0000004800.57449.67. Pituitary. 2003. PMID: 14703019 Review.
Cited by
-
Association of growth hormone deficiency with an increased number of preadipocytes in subcutaneous fat.Front Endocrinol (Lausanne). 2023 May 26;14:1199589. doi: 10.3389/fendo.2023.1199589. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37305046 Free PMC article.
References
-
- Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P. Impact of growth hormone (Gh) treatment on cardiovascular risk factors in gh-deficient adults: A metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab (2004) 89(5):2192–9. doi: 10.1210/jc.2003-030840 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

