Increased plasma genistein after bariatric surgery could promote remission of NAFLD in patients with obesity
- PMID: 36686492
- PMCID: PMC9846086
- DOI: 10.3389/fendo.2022.1024769
Increased plasma genistein after bariatric surgery could promote remission of NAFLD in patients with obesity
Abstract
Background: Bariatric surgery is associated with a positive effect on the progress of non-alcoholic associated fatty liver disease (NAFLD). Although weight loss is the obvious mechanism, there are also weight-independent mechanisms.
Methods: We collected blood samples from 5 patients with obesity before and 3 months after surgery and performed an LC-MS-based untargeted metabolomics test to detect potential systemic changes. We also constructed sleeve gastrectomy (SG) mice models. The plasma, liver and intestine samples were collected and analyzed by qPCR, ELISA and HPLC. Cohousing experiments and feces transplantation experiments were performed on mice to study the effect of gut microbiota. Genistein administration experiments were used to study the in vivo function of the metabolites.
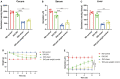
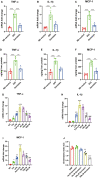
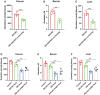
Results: Plasma genistein (GE) was identified to be elevated after surgery. Both clinical data and rodent models suggested that plasma GE is negatively related to the degree of NAFLD. We fed diet-induced obese (DIO) mice with GE, and we found that there was significant remission of NAFLD. Both in vivo and in vitro experiments showed that GE could restrict the inflammation state in the liver and thus relieve NAFLD. Finally, we used co-housing experiments to alter the gut microbiota in mice, and it was identified that sleeve gastrectomy (SG) mice had a special gut microbiota phenotype, which could result in higher plasma GE levels. By feces transplantation experiment (FMT), we found that only feces from the SG mice (and not from other lean mice) could induce higher plasma GE levels.
Conclusion: Our studies showed that SG but not calorie restriction could induce higher plasma GE levels by altering the gut microbiota. This change could promote NAFLD remission. Our study provides new insights into the systemic effects of bariatric surgery. Bariatric surgery could affect remote organs via altered metabolites from the gut microbiota. Our study also identified that additional supplement of GE after surgery could be a therapy for NAFLD.
Keywords: bariatric surgery; genistein; gut microbiota; non-alcoholic fatty liver disease; obese; sleeve gastrectomy.
Copyright © 2023 Wang, Wang, Bai, Li, Liu, Deng, Zhou, Tao and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Microbial changes from bariatric surgery alters glucose-dependent insulinotropic polypeptide and prevents fatty liver disease.Gut Microbes. 2023 Jan-Dec;15(1):2167170. doi: 10.1080/19490976.2023.2167170. Gut Microbes. 2023. PMID: 36732495 Free PMC article.
-
Short-term changes in the serum metabolome after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass.Metabolomics. 2021 Aug 5;17(8):71. doi: 10.1007/s11306-021-01826-y. Metabolomics. 2021. PMID: 34355282
-
Morphofunctional Changes After Sleeve Gastrectomy and Very Low Calorie Diet in an Animal Model of Non-Alcoholic Fatty Liver Disease.Obes Surg. 2018 Jan;28(1):142-151. doi: 10.1007/s11695-017-2805-4. Obes Surg. 2018. PMID: 28710554
-
Gut microbiota and related metabolites in the pathogenesis of nonalcoholic steatohepatitis and its resolution after bariatric surgery.Obes Rev. 2022 Feb;23(2):e13367. doi: 10.1111/obr.13367. Epub 2021 Nov 2. Obes Rev. 2022. PMID: 34729904 Review.
-
Bariatric Surgery Improves Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis.Obes Surg. 2022 Jun;32(6):1872-1883. doi: 10.1007/s11695-022-06011-1. Epub 2022 Apr 6. Obes Surg. 2022. PMID: 35386040
Cited by
-
Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations.Biology (Basel). 2024 Apr 6;13(4):243. doi: 10.3390/biology13040243. Biology (Basel). 2024. PMID: 38666855 Free PMC article. Review.
-
Advances in Mass Spectrometry-Based Blood Metabolomics Profiling for Non-Cancer Diseases: A Comprehensive Review.Metabolites. 2024 Jan 14;14(1):54. doi: 10.3390/metabo14010054. Metabolites. 2024. PMID: 38248857 Free PMC article. Review.
-
Impact of bariatric surgery on gut microbiota in obese patients: A systematic review.Indian J Gastroenterol. 2025 Aug;44(4):457-477. doi: 10.1007/s12664-025-01763-x. Epub 2025 Apr 12. Indian J Gastroenterol. 2025. PMID: 40220249
-
Sleeve Gastrectomy Protects Against Hypertension in Rats due to Changes in the Gut Microbiome.J Surg Res. 2024 Sep;301:118-126. doi: 10.1016/j.jss.2024.05.044. Epub 2024 Jun 25. J Surg Res. 2024. PMID: 38925098 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

