Tenofovir use is associated with a decreased risk of hepatocellular carcinoma among men with HIV irrespective of coinfection status
- PMID: 36686591
- PMCID: PMC9852951
- DOI: 10.1016/j.jhepr.2022.100634
Tenofovir use is associated with a decreased risk of hepatocellular carcinoma among men with HIV irrespective of coinfection status
Abstract
Background & aims: Tenofovir is recommended as part of the first-line antiretroviral therapy (ART) to treat people living with HIV (PLWH) with HBV coinfection. However, the effects of tenofovir-containing ART on hepatocellular carcinoma (HCC) risk among PLWH with/without chronic hepatitis virus infections remain unclear.
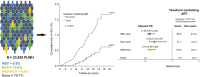
Methods: This study included 23,838 PLWH. All of them were males aged ≥20 years and followed prospectively during 2000-2017. Four major nationwide registries - the Human Immunodeficiency Virus surveillance database, Taiwan Cancer Registry, Death Certification System, and National Health Insurance Database - were applied to define ART and comorbidities and ascertain newly diagnosed HCC. Tenofovir-containing ART was identified through prescription records. Cox proportional hazards models were used to determine the association of tenofovir use with HCC incidence.
Results: HCC incidence was lower among ever users of tenofovir than among never users (24.2 and 85.7 per 100,000 person-years, respectively). Ever users had significantly reduced HCC risk (adjusted hazard ratio 0.20, 95% CI 0.13-0.31). The effect of tenofovir use on reduced risk for HCC consistently favored never users across many prespecified subgroups, including HBV or HCV coinfection (p <0.05). The findings were consistent in subgroups of PLWH diagnosed with HIV before tenofovir's approval and in those born before the nationwide roll-out of neonatal HBV vaccination.
Conclusions: Our findings underscore the need for randomized controlled trials of tenofovir in combination with long-acting injectable ART regimens to assess its safety and efficacy in PLWH, particularly in those with HBV or HCV coinfection.
Impact and implications: Tenofovir's effect on the risk of hepatocellular carcinoma (HCC) among people living with HIV with hepatitis B or C coinfection remains under investigated. This nationwide prospective cohort study, comprising 23,838 men living with HIV, showed that tenofovir-containing antiretroviral therapy was associated with reduced risk of HCC (adjusted relative risk: 0.20, 95% CI 0.13-0.31), which was consistently observed across many prespecified subgroups. The effect of tenofovir use on HCC risk should be further investigated in PLWH, particularly following the development of long-acting injectable ART regimens.
Keywords: AIDS; AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HCC, hepatocellular carcinoma; HRs, hazard ratios; NHI, National Health Insurance; PLWH, people living with HIV; chronic hepatitis; nationwide registry; prospective study; risk assessment.
© 2022 The Author(s).
Conflict of interest statement
The authors disclose no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Platt L., Easterbrook P., Gower E., McDonald B., Sabin K., McGowan C., et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797–808. - PubMed
-
- Rosenthal E., Roussillon C., Salmon-Ceron D., Georget A., Henard S., Huleux T., et al. Liver-related deaths in HIV-infected patients between 1995 and 2010 in France: the Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalite 2010 survey. HIV Med. 2015;16:230–239. - PubMed
LinkOut - more resources
Full Text Sources

