Comparative efficacy of Chinese patent medicines for non-alcoholic fatty liver disease: A network meta-analysis
- PMID: 36686656
- PMCID: PMC9847677
- DOI: 10.3389/fphar.2022.1077180
Comparative efficacy of Chinese patent medicines for non-alcoholic fatty liver disease: A network meta-analysis
Abstract
Background: The incidence of Non-alcoholic fatty liver disease (NAFLD) is increasing year by year. Researches showed that Chinese patent medicines (CPMs) had achieved good efficacy in the treatment of Non-alcoholic fatty liver disease. However, the debate on optimum Chinese patent medicine (CPM) persists. Therefore, we conducted a network meta-analysis to objectively compare the efficacy of different Chinese patent medicines in the treatment of Non-alcoholic fatty liver disease. Methods: PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wanfang Database, China Science and Technology Journal Database, and Chinese Biomedical Literature Database were used as databases for RCT researches retrieval. The retrieval time was from establishment of the database to July 2022. After effective data was extracted, Review Manager 5.4 and Cochrane Collaboration System Evaluator's Manual were used to assess bias risk. STATA 16.0 based on frequency theory was used for the network meta-analysis. Results: Totally 39 studies were included, involving 13 Chinese patent medicines, including 4049 patients, of which 42 patients were lost. In terms of improving clinical efficiency rate, Zhibitai capsule was most likely the best choice of Chinese patent medicine for Non-alcoholic fatty liver disease. Liuwei Wuling tablet had the best effect in reducing serum ALT and AST; Gandan Shukang capsule had the best effect in reducing serum GGT; Qianggan capsule had the best effect in reducing serum TG; Dangfei Liganning capsule had the best effect in reducing serum TC. None of the included studies had serious adverse reactions. Conclusion: For patients with Non-alcoholic fatty liver disease in this NMA, Zhibitai capsule, Liuwei Wuling tablet, Gandan Shukang capsule, Qianggan capsule, Dangfei Liganning capsule might be noteworthy. Due to the uclear risk bias, better designed double-blind, multi center and large sample RCTs are needed which resolve the problems of blinding, selective reporting and allocation concealment. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022341240.
Keywords: Chinese patent medicine (CPM); efficacy; network meta-analysis; non-alcoholic fatty liver disease; western medicine.
Copyright © 2023 Zhang, Liu, Gu, Li, Liu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
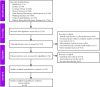
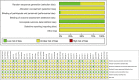
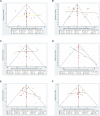
Figures


Similar articles
-
Chinese patent medicines combined with hormone replacement therapy for premature ovarian failure: A Bayesian network meta-analysis.Front Med (Lausanne). 2022 Nov 17;9:1043390. doi: 10.3389/fmed.2022.1043390. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36465907 Free PMC article.
-
Efficacy and safety of oral Chinese patent medicine for benign prostatic hyperplasia: a network meta-analysis of randomized controlled trials.Front Med (Lausanne). 2024 Dec 27;11:1483864. doi: 10.3389/fmed.2024.1483864. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39802886 Free PMC article.
-
Comparative efficacy of eight oral Chinese patent medicines for dilated cardiomyopathy with heart failure: a Bayesian network meta-analysis.Syst Rev. 2024 Aug 31;13(1):222. doi: 10.1186/s13643-024-02582-5. Syst Rev. 2024. PMID: 39217375 Free PMC article.
-
Efficacy and safety of Chinese patent medicines combined with conventional therapies for endometriosis: A systematic review and bayesian network meta-analysis.J Ethnopharmacol. 2025 Mar 13;343:119465. doi: 10.1016/j.jep.2025.119465. Epub 2025 Feb 8. J Ethnopharmacol. 2025. PMID: 39929398
-
The comparative effects of oral Chinese patent medicines combined with western medicine in stable angina: A systematic review and network meta-analysis of 179 trials.Front Pharmacol. 2022 Aug 17;13:918689. doi: 10.3389/fphar.2022.918689. eCollection 2022. Front Pharmacol. 2022. PMID: 36059992 Free PMC article.
Cited by
-
Comparative efficacy of topical commercial Chinese polyherbal preparation for vulvovaginal candidiasis: a network meta-analysis.Front Pharmacol. 2025 Feb 3;16:1484325. doi: 10.3389/fphar.2025.1484325. eCollection 2025. Front Pharmacol. 2025. PMID: 39963237 Free PMC article.
References
-
- Chen Z. X., Zhang S. J., Yun L. R. (2006). Clinical study of treatment nonalcoholic fatty liver with qianggan capsule. Zhongguo Zhong Yao Za Zhi 31 (20), 1739–1741. - PubMed
-
- Cipriani A., Furukawa T. A., Salanti G., Chaimani A., Atkinson L. Z., Ogawa Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 391 (10128), 1357–1366. 10.1016/s0140-6736(17)32802-7 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

