MRI-based multiregional radiomics for predicting lymph nodes status and prognosis in patients with resectable rectal cancer
- PMID: 36686763
- PMCID: PMC9846353
- DOI: 10.3389/fonc.2022.1087882
MRI-based multiregional radiomics for predicting lymph nodes status and prognosis in patients with resectable rectal cancer
Abstract
Purpose: To establish and evaluate multiregional T2-weighted imaging (T2WI)-based clinical-radiomics model for predicting lymph node metastasis (LNM) and prognosis in patients with resectable rectal cancer.
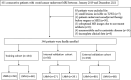
Methods: A total of 346 patients with pathologically confirmed rectal cancer from two hospitals between January 2019 and December 2021 were prospectively enrolled. Intra- and peritumoral features were extracted separately, and least absolute shrinkage and selection operator regression was applied for feature selection. Radiomics signatures were built using the selected features from different regions. The clinical-radiomic nomogram was developed by combining the intratumoral and peritumoral radiomics signatures score (radscore) and the most predictive clinical parameters. The diagnostic performances of the nomogram and clinical model were evaluated using the area under the receiver operating characteristic curve (AUC). The prognostic model for 3-year recurrence-free survival (RFS) was constructed using univariate and multivariate Cox analysis.
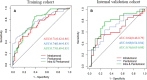
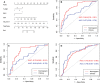
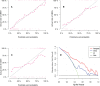
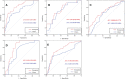
Results: The intratumoral radscore (radscore 1) included four features, the peritumoral radscore (radscore 2) included five features, and the combined intratumoral and peritumoural radscore (radscore 3) included ten features. The AUCs for radscore 3 were higher than that of radscore 1 in training cohort (0.77 vs. 0.71, P=0.182) and internal validation cohort (0.76 vs. 0.64, P=0.041). The AUCs for radscore 3 were higher than that of radscore 2 in training cohort (0.77 vs. 0.74, P=0.215) and internal validation cohort (0.76 vs. 0.68, P=0.083). A clinical-radiomic nomogram showed a higher AUC compared with the clinical model in training cohort (0.84 vs. 0.67, P<0.001) and internal validation cohort (0.78 vs. 0.64, P=0.038) but not in external validation (0.72 vs. 0.76, P=0.164). Multivariate Cox analysis showed MRI-reported extramural vascular invasion (EMVI) (HR=1.099, 95%CI: 0.462-2.616; P=0.031) and clinical-radiomic nomogram-based LNM (HR=2.232, 95%CI:1.238-7.439; P=0.017) were independent risk factors for assessing 3-year RFS. Combined clinical-radiomic nomogram based LNM and MRI-reported EMVI showed good performance in training cohort (AUC=0.748), internal validation cohort (AUC=0.706) and external validation (AUC=0.688) for predicting 3-year RFS.
Conclusion: A clinical-radiomics nomogram exhibits good performance for predicting preoperative LNM. Combined clinical-radiomic nomogram based LNM and MRI-reported EMVI showed clinical potential for assessing 3-year RFS.
Keywords: lymph node; magnetic resonance imaging; prognosis; radiomics; rectal neoplasms.
Copyright © 2023 Li, Chen, Liu, Lu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
MRI-based multiregional radiomics for preoperative prediction of tumor deposit and prognosis in resectable rectal cancer: a bicenter study.Eur Radiol. 2023 Nov;33(11):7561-7572. doi: 10.1007/s00330-023-09723-9. Epub 2023 May 9. Eur Radiol. 2023. PMID: 37160427
-
T2WI-based MRI radiomics for the prediction of preoperative extranodal extension and prognosis in resectable rectal cancer.Insights Imaging. 2024 Feb 27;15(1):57. doi: 10.1186/s13244-024-01625-8. Insights Imaging. 2024. PMID: 38411722 Free PMC article.
-
Preoperative prediction of lymph node metastasis in endometrial cancer patients via an intratumoral and peritumoral multiparameter MRI radiomics nomogram.Front Oncol. 2024 Sep 19;14:1472892. doi: 10.3389/fonc.2024.1472892. eCollection 2024. Front Oncol. 2024. PMID: 39364314 Free PMC article.
-
Predicting clinically significant prostate cancer in PI-RADS 3 lesions using MRI-based radiomics: a literature review of methodological variations and performance.Abdom Radiol (NY). 2025 Apr 2. doi: 10.1007/s00261-025-04914-y. Online ahead of print. Abdom Radiol (NY). 2025. PMID: 40172658 Review.
-
Preoperative magnetic resonance imaging-radiomics in cervical cancer: a systematic review and meta-analysis.Front Oncol. 2024 Jul 4;14:1416378. doi: 10.3389/fonc.2024.1416378. eCollection 2024. Front Oncol. 2024. PMID: 39026971 Free PMC article.
Cited by
-
Beyond the tumor region: Peritumoral radiomics enhances prognostic accuracy in locally advanced rectal cancer.World J Gastroenterol. 2025 Feb 28;31(8):99036. doi: 10.3748/wjg.v31.i8.99036. World J Gastroenterol. 2025. PMID: 40062323 Free PMC article.
-
MRI T2WI-based radiomics combined with KRAS gene mutation constructed models for predicting liver metastasis in rectal cancer.BMC Med Imaging. 2024 Oct 4;24(1):262. doi: 10.1186/s12880-024-01439-6. BMC Med Imaging. 2024. PMID: 39367333 Free PMC article.
-
Comparison of preoperative CT- and MRI-based multiparametric radiomics in the prediction of lymph node metastasis in rectal cancer.Front Oncol. 2023 Nov 24;13:1230698. doi: 10.3389/fonc.2023.1230698. eCollection 2023. Front Oncol. 2023. PMID: 38074652 Free PMC article.
-
Diagnostic performance of Node-RADS score for mesorectal lymph node metastasis in rectal cancer.Abdom Radiol (NY). 2025 Jan;50(1):38-48. doi: 10.1007/s00261-024-04497-0. Epub 2024 Jul 24. Abdom Radiol (NY). 2025. PMID: 39046482
-
Artificial intelligence-driven radiomics study in cancer: the role of feature engineering and modeling.Mil Med Res. 2023 May 16;10(1):22. doi: 10.1186/s40779-023-00458-8. Mil Med Res. 2023. PMID: 37189155 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

