Fabrication of bio-inspired metal-based superhydrophilic and underwater superoleophobic porous materials by hydrothermal treatment and magnetron sputtering
- PMID: 36686915
- PMCID: PMC9811985
- DOI: 10.1039/d2ra07113d
Fabrication of bio-inspired metal-based superhydrophilic and underwater superoleophobic porous materials by hydrothermal treatment and magnetron sputtering
Abstract
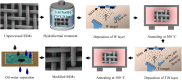
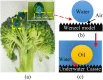
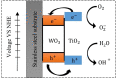
Oil-water separation using porous superhydrophilic materials is a promising method to circumvent the issue of oil-polluted water by separating water from oil-water mixtures. However, fabricating metal-based porous superhydrophilic materials with stable superhydrophilicity that can recover their strong hydrophilicity and have acceptable oil-water separation efficiency without complex external stimuli is still a challenge. Inspired by the anti-wetting behavior of broccoli buds, this study successfully fabricated metal-based superhydrophilic and underwater superoleophobic porous materials by hydrothermal treatment of stainless steel meshes (SSMs) combined with magnetron sputtering of metallic Ti and W. The process was then followed with annealing at 300 °C for 4 hours. The effects of coating materials, annealing temperature, and surface structure on the wetting behavior of the prepared meshes were studied and analyzed. The modified meshes exhibited unique broccoli-like microstructures coated with thin TiO2-x N x /WO3 films and showed superhydrophilicity with a 0° water contact angle (WCA) and underwater superoleophobicity with underwater oil contact angles (UOCAs) higher than 155°. They also maintained strong hydrophilicity for more than three weeks with WCAs of less than 13°. Besides, they could recover their initial superhydrophilicity with a 0° WCA after post-annealing at 80 °C for 30 minutes. Notably, the broccoli-like structures and the strong hydrophilic coatings contributed to a significant water flow rate (Q) of 3650 L m-2 h-1 and satisfactory oil-water separation efficiency of 98% for more than 15 separation cycles toward various oil-water mixtures. We believe that the presented method and fabricated material are promising and can be applied to induce hydrophilicity of various metallic materials for practical applications of oil-water separation, anti-fouling, microfluidic transport, and water harvesting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
Figures





Similar articles
-
Micro/Nanoscale Structured Superhydrophilic and Underwater Superoleophobic Hybrid-Coated Mesh for High-Efficiency Oil/Water Separation.Polymers (Basel). 2020 Jun 19;12(6):1378. doi: 10.3390/polym12061378. Polymers (Basel). 2020. PMID: 32575503 Free PMC article.
-
Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation.Polymers (Basel). 2023 Dec 19;16(1):14. doi: 10.3390/polym16010014. Polymers (Basel). 2023. PMID: 38201679 Free PMC article.
-
Preparation of Superhydrophilic/Underwater Superoleophobic and Superhydrophobic Stainless Steel Meshes Used for Oil/Water Separation.Polymers (Basel). 2023 Jul 14;15(14):3042. doi: 10.3390/polym15143042. Polymers (Basel). 2023. PMID: 37514432 Free PMC article.
-
Nacre-inspired underwater superoleophobic films with high transparency and mechanical robustness.Nat Protoc. 2022 Nov;17(11):2647-2667. doi: 10.1038/s41596-022-00725-3. Epub 2022 Aug 15. Nat Protoc. 2022. PMID: 35970874 Review.
-
Superwetting Polymeric Three Dimensional (3D) Porous Materials for Oil/Water Separation: A Review.Polymers (Basel). 2019 May 6;11(5):806. doi: 10.3390/polym11050806. Polymers (Basel). 2019. PMID: 31064062 Free PMC article. Review.
References
-
- Cheryan M. Rajagopalan N. J. Membr. Sci. 2015;151:13–28.
-
- Gong Z. Yang N. Chen Z. Jiang B. Sun Y. Yang X. Zhang L. Chem. Eng. J. 2020;380:122524.
-
- Ha K. H. Chu C. N. J. Micromech. Microeng. 2016;26:045008.
-
- Zhang L. Yang X. Jiang B. Sun Y. Gong Z. Zhang N. Hou S. Li J. Yang N. Appl. Surf. Sci. 2018;456:114–123.
-
- Zhou C. Li Y. Li H. Zeng X. Pi P. Wen X. Xu S. Cheng J. Surf. Coat. Technol. 2017;31:55–62.
LinkOut - more resources
Full Text Sources

