Computational study of linear carbon chain based organic dyes for dye sensitized solar cells
- PMID: 36686920
- PMCID: PMC9811357
- DOI: 10.1039/d2ra06767f
Computational study of linear carbon chain based organic dyes for dye sensitized solar cells
Erratum in
-
Correction: Computational study of linear carbon chain based organic dyes for dye sensitized solar cells.RSC Adv. 2024 Apr 11;14(17):11676. doi: 10.1039/d4ra90037e. eCollection 2024 Apr 10. RSC Adv. 2024. PMID: 38605899 Free PMC article.
Abstract
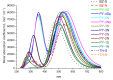
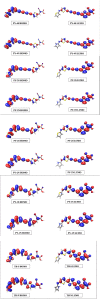
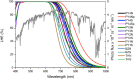
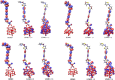
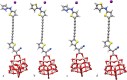
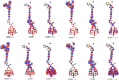
Spectroscopic, electronic and electron injection properties of a new class of linear carbon chain (LCC) based organic dyes have been investigated, by means of density functional theory (DFT) and time-dependent density functional theory (TDDFT), for application in dye-sensitized solar cells (DSSCs). The photophysical properties of LCC-based dyes are tuned by changing the length of the linear carbon chain; UV/VIS absorption is red-shifted with increasing LCC length whereas oscillator strength and electron injection properties are reduced. Excellent nonlinear optical properties are predicted in particular for PY-N4 and PY-S4 dyes in the planar conformation. Results indicate that a LCC-bridge produces better results compared to benzene and thiophene bridges. Simulations of I--Dye@(TiO2)14 and Dye@(TiO2)14 anatase complexes indicate that designed dyes inject electrons efficiently into the TiO2 surface and can be regenerated by electron transfer from the electrolyte. Superior properties in terms of efficiency are shown by compounds with a pyrrole ring as the donor group and PY-3N is expected to be a promising candidate for applications, however all the investigated dyes could provide a good performance in solar energy conversion. Our study demonstrates that computational design can provide a significant contribution to experimental work; we expect this study will contribute to future developments to identify new and highly efficient sensitizers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Predicting the dye-sensitized solar cell performance of novel linear carbon chain-based dyes: insights from DFT simulations.Dalton Trans. 2023 Nov 7;52(43):15995-16004. doi: 10.1039/d3dt01856c. Dalton Trans. 2023. PMID: 37847522
-
Modification of benzoindenothiophene-based organic dye with fused thiophenes for efficient dye-sensitized solar cells.J Mol Graph Model. 2022 Sep;115:108214. doi: 10.1016/j.jmgm.2022.108214. Epub 2022 May 14. J Mol Graph Model. 2022. PMID: 35598558
-
Theoretical investigation of the effect of changing the auxiliary acceptor on the performance of organic D-A'-A dyes used as sensitizers in DSSCs.J Mol Model. 2023 Nov 10;29(12):365. doi: 10.1007/s00894-023-05766-3. J Mol Model. 2023. PMID: 37946060
-
Electronic and optical properties of dye-sensitized TiO₂ interfaces.Top Curr Chem. 2014;347:1-45. doi: 10.1007/128_2013_507. Top Curr Chem. 2014. PMID: 24488437
-
Recent Advances in the Application of Coumarins as Photosensitizers for the Construction of a Dye-Sensitized Solar Cell.ACS Omega. 2025 Apr 1;10(14):13726-13748. doi: 10.1021/acsomega.4c11135. eCollection 2025 Apr 15. ACS Omega. 2025. PMID: 40256496 Free PMC article. Review.
Cited by
-
Optimizing photovoltaic performance of squaraine derivative dyes: a DFT study on different anchoring groups.RSC Adv. 2024 Aug 2;14(33):24185-24195. doi: 10.1039/d4ra05322b. eCollection 2024 Jul 26. RSC Adv. 2024. PMID: 39101065 Free PMC article.
-
Nano-Hybrid Ag@LCCs Systems with Potential Wound-Healing Properties.Materials (Basel). 2023 Mar 18;16(6):2435. doi: 10.3390/ma16062435. Materials (Basel). 2023. PMID: 36984315 Free PMC article.
References
-
- O'Regan B. Grätzel M. Nature. 1991;353:737–740.
-
- Parisi M. L. Maranghi S. Basosi R. Renew. Sustain. Energy Rev. 2014;39:124–138.
-
- Zhou X. Zhang Y. Porter A. L. Guo Y. Zhu D. Scientometrics. 2014;100:705–721.
-
- Shalini S. Balasundaraprabhu R. Satish Kumar T. Prabavathy N. Senthilarasu S. Prasanna S. Int. J. Energy Res. 2016;40:1303–1320.
-
- Nazeeruddin M. K. Péchy P. Grätzel M. Chem. Commun. 1997;1705:1705–1706.
LinkOut - more resources
Full Text Sources

