Ligand-assisted morphology regulation of AuNi bimetallic nanocrystals for efficient hydrogen evolution
- PMID: 36686932
- PMCID: PMC9812016
- DOI: 10.1039/d2ra06325e
Ligand-assisted morphology regulation of AuNi bimetallic nanocrystals for efficient hydrogen evolution
Abstract
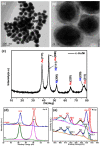
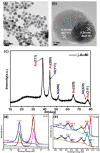
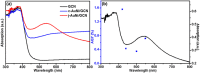
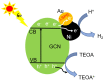
We report the controllable synthesis of AuNi core-shell (c-AuNi) and Janus (j-AuNi) nanocrystals (NCs) with uniform shape, tunable size and compositions in the presence of trioctylphosphine (TOP) or triphenylphosphine (TPP). The morphology of the AuNi bimetallic NCs could be regulated by varying the structure and concentration of phosphine ligands. The ligand-directed structural evolution mechanism of AuNi bimetallic NCs was investigated and discussed in detail. When loaded on graphitic carbon nitride (GCN) for photocatalytic hydrogen generation, the obtained j-AuNi NCs showed much higher activity for hydrogen evolution than the monometallic (Au and Ni) counterparts, owing to the synergistic effect of plasmon enhanced light absorption from the Au portion and additional electron sink effect from the Ni portion. This work provides a promising route for preparing low-cost Au-based bimetallic catalysts with controllable morphologies and high activities for hydrogen production.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Shape-controlled synthesis of Au-Pd bimetallic nanocrystals for catalytic applications.Chem Soc Rev. 2016 Jul 21;45(14):3916-34. doi: 10.1039/c5cs00958h. Epub 2016 Apr 20. Chem Soc Rev. 2016. PMID: 27095006
-
Aqueous Topological Synthesis of Au@semiconductor Core-Shell Nanocrystals with Morphology and Composition Engineering.Inorg Chem. 2024 Jun 3;63(22):10358-10365. doi: 10.1021/acs.inorgchem.4c01238. Epub 2024 May 20. Inorg Chem. 2024. PMID: 38767279
-
Bimetallic Ni-Co nanoparticles confined within nitrogen defective carbon nitride nanotubes for enhanced photocatalytic hydrogen production.Environ Res. 2022 Jan;203:111844. doi: 10.1016/j.envres.2021.111844. Epub 2021 Aug 5. Environ Res. 2022. PMID: 34364861
-
Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing.Nanomaterials (Basel). 2021 Jul 26;11(8):1926. doi: 10.3390/nano11081926. Nanomaterials (Basel). 2021. PMID: 34443756 Free PMC article. Review.
-
Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications.Adv Mater. 2011 Mar 4;23(9):1044-60. doi: 10.1002/adma.201003695. Epub 2011 Jan 7. Adv Mater. 2011. PMID: 21218429 Review.
Cited by
-
Controlled Synthesis of Mesoporous Solid Polymer Electrolyte Au(Pt)NiCe/C Membrane Electrode for Electrocatalytic Hydrogenation.Micromachines (Basel). 2025 Apr 3;16(4):436. doi: 10.3390/mi16040436. Micromachines (Basel). 2025. PMID: 40283311 Free PMC article.
-
Plasmonic Nanomaterials for Versatile Solar Energy Conversion Applications.ACS Omega. 2025 Jul 7;10(27):28615-28629. doi: 10.1021/acsomega.5c02075. eCollection 2025 Jul 15. ACS Omega. 2025. PMID: 40687009 Free PMC article. Review.
References
-
- Wang C. Astruc D. Chem. Soc. Rev. 2014;43:7188–7216. - PubMed
-
- Rayalu S. S. Jose D. Joshi M. V. Mangrulkar P. A. Shrestha K. Klabunde K. Appl. Catal., B. 2013;142:684–693.
-
- Tanaka A. Hashimoto K. Kominami H. J. Am. Chem. Soc. 2012;134:14526–14533. - PubMed
-
- Li B. Zhang B. Nie S. Shao L. Hu L. J. Catal. 2017;348:256–264.
LinkOut - more resources
Full Text Sources

