New boro amino amide organocatalysts for asymmetric cross aldol reaction of ketones with carbonyl compounds
- PMID: 36686933
- PMCID: PMC9811241
- DOI: 10.1039/d2ra06272k
New boro amino amide organocatalysts for asymmetric cross aldol reaction of ketones with carbonyl compounds
Abstract
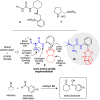
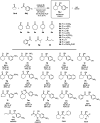
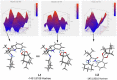
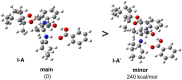
Distinct types of new boron fused primary amino amide organocatalysts were designed and synthesized from commercially available amino acids. Their catalytic activities were investigated in asymmetric crossed aldol reaction of ketones with aromatic aldehydes to afford the corresponding chiral anti-aldol adducts with good chemical yields, moderate diastereoselectivity and good to excellent enantioselectivities (up to 94% yields, up to 90 : 10 dr, up to 94% ee).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Tang Z. Jiang F. Yu L. T. Cui X. Gong L. Z. Mi A. Q. Jiang Y. Z. Wu Y. D. J. Am. Chem. Soc. 2003;125:5262. - PubMed
- Chi Y. Gellman S. H. Org. Lett. 2005;7:4253. - PubMed
- Lattanzi A. Org. Lett. 2005;7:2579. - PubMed
- chimni S. S. Mahajan D. Tetrahedron: Asymmetry. 2006;17:2108.
- De Figueiredo R. M. Christmann M. Eur. J. Org. Chem. 2007;16:2575.
- Enders D. Grondal C. Matthias R. M. Angew. Chem. 2007;46:1570. - PubMed
- MacMillan D. W. C. Nature. 2008;455:304. - PubMed
- Bertelsen S. Jorgensen K. A. Chem. Soc. Rev. 2009;38:2178. - PubMed
- Moyano A. Rios R. Chem. Rev. 2011;111:4703. - PubMed
- Kasaplar P. Escrich C. R. Pericas M. A. Org. Lett. 2013;15:3498. - PubMed
- SIlverio D. L. Torker S. Pilyugina T. Vieria E. M. Snapper M. L. Haeffener F. Hoyeda A. H. Nature. 2013;494:7436. - PMC - PubMed
- He H. X. Du D. M. RSC. Adv. 2013;3:16349.
- Saravanan S. Khan N. H. Kureshy R. I. Abdi S. H. Bajaj H. C. ACS Catal. 2013;3:2873.
- Volla C. M. R. Atodiresei I. Rueping M. Chem. Rev. 2014;114:2390. - PubMed
- Fang X. Wang C. J. Chem. Commun. 2015;51:1185. - PubMed
- Chen J. Meng S. Wang L. Tang H. Huang Y. Chem. Sci. 2015;6:4184. - PMC - PubMed
- Xu L. Huang J. Liu Y. Wang Y. Xu B. Ding K. Ding Y. Xu Q. Yu L. Fan Y. RSC Adv. 2015;5:42178.
- Sekikawa T. Kitaguchi T. Kitaura H. Minami T. Hatanaka Y. Org. Lett. 2016;18:646. - PubMed
- Varal U. Durmaz M. Sirit A. Org. Chem. Front. 2016;3:730.
- Kaur J. Chauhan P. Chimni S. S. Org. Biomol. Chem. 2016;14:7832. - PubMed
- Evans C. S. Davis L. O. Molecules. 2018;23:33.
- Zhang H. Han M. Chen T. Xu L. Yu L. RSC Adv. 2017;7:48214.
- Zheng Y. Wu A. Ke Y. Cao H. Yu L. Chin. Chem. Lett. 2019;30:937.
- Xiang S. H. Tan B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 2020;11:3786. - PMC - PubMed
-
- Nakagawa M. Nakano H. Watanabe K.-I. Chem. Lett. 1985:391.
- Yamada Y. Watanabe K.-I. Yasuda H. Utsunomiya Daigaku Kyoikugakubu Kiyo, Dai-2-bu. 1989;39:25.
- Yamada Y. M. A. Yoshikawa N. Sasai H. Shibasaki M. Angew. Chem., Int. Ed. Engl. 1997;36:1871.
- Yamada Y. M. A. Shibasaki M. Tetrahedron Lett. 1998;39:5561.
-
- List B. Lerner R. A. Barbas III C. F. J. Am. Chem. Soc. 2000;122:2395.
-
- Mukherjee S. Yang J. W. Hoffmann S. List B. Chem. Rev. 2007;107:5471. - PubMed
- Notz W. Tanaka F. Barbas III C. F. Acc. Chem. Res. 2004;37:580. - PubMed
- Zhang S. J. Xie H. X. Zhu J. Li H. Zhang X. S. Li J. Wang W. Nat. Commun. 2011:211. - PubMed
- Zou Y. Q. Hormann F. M. Bach T. Chem. Soc. Rev. 2018;47:278. - PMC - PubMed
- Fang X. Wang C. J. Chem. Commun. 2015;51:1185. - PubMed
- Yu X. Wang W. Chem.–Asian J. 2008;3:516. - PubMed
- Bae H. Y. Some S. Soh J. Lee Y. S. Song C. E. Chem. Commun. 2011;47:9621. - PubMed
-
- Shang M. Wang X. Koo S. M. Youn J. Chan J. Z. Yao W. Hastings B. T. Wasa M. J. Am. Chem. Soc. 2017;139:95. - PubMed
- Matsui K. Takizawa S. Sasai H. Synlett. 2006;5:761.
- Schenker S. Zamfir A. Fraund M. Tsogoeva S. B. Eur. J. Org. Chem. 2011;12:2209.
- Zhang Z. Bae H. Y. Guin J. Rabalakos C. Genmeren M. V. Leutzsch M. Klussmann M. List B. Nat. Commun. 2016:12478. - PMC - PubMed
- Hirashima S. Yamamoto H. J. Synth. Org. Chem., Jpn. 2013;71:1116.
LinkOut - more resources
Full Text Sources

