Circular RNA SMARCA5 Modulates Epithelial-Mesenchymal Transformation, Proliferation, and Metastasis of Nasopharyngeal Carcinoma Cells via microRNA-582-3p/Phosphatase and Tensin Homolog Axis
- PMID: 36686977
- PMCID: PMC9859696
- DOI: 10.1155/2023/5177471
Circular RNA SMARCA5 Modulates Epithelial-Mesenchymal Transformation, Proliferation, and Metastasis of Nasopharyngeal Carcinoma Cells via microRNA-582-3p/Phosphatase and Tensin Homolog Axis
Retraction in
-
Retracted: Circular RNA SMARCA5 Modulates Epithelial-Mesenchymal Transformation, Proliferation, and Metastasis of Nasopharyngeal Carcinoma Cells via microRNA-582-3p/Phosphatase and Tensin Homolog Axis.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9876258. doi: 10.1155/2023/9876258. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125158 Free PMC article.
Abstract
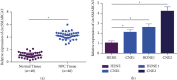
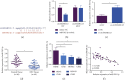
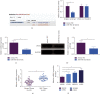
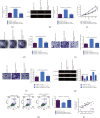
The action mechanism in which circular RNA (circ) SMARCA5 targeted nasopharyngeal carcinoma (NPC) cell proliferation, migration, invasion, and apoptosis via microRNA (miR)-582-3p/phosphatase and tensin homolog (PTEN) axis was explored. The examination was performed via reverse transcription-quantitative polymerase chain reaction (RT-qPCR), discovering that circSMARCA5 was elevated while miR-582-3p was silenced in NPC tissues and cells. E-cadherin and N-cadherin were detected. The results illustrated transfection with si-circSMARCA5 or miR-582-3p-mimic was available to repress cancer cell advancement, and E-cadherin was augmented. Transfection with pcDNA 3.1-circSMARCA5 or miR-582-3p-inhibitor was available to accelerate cancer cell advancement, and N-cadherin was augmented. MiR-582-3p-inhibitor blocked the suppression of si-circSMARCA5 on NPC. The si-PTEN blocked the malignant behavior of pcDNA 3.1-circSMARCA5 against NPC. The binding sites between circSMARCA5 and miR-582-3p and between miR-582-3p and PTEN were verified. Linear analysis results illuminated the expression pattern of circSMARCA5 was opposite to miR-582-3p, while the expression pattern of circSMARCA5 was positively associated with PTEN. In brief, the results of the research clarified circSMARCA5 modulated NPC cells' vital movement via the miR-582-3p/PTEN molecular axis.
Copyright © 2023 Hui Wang and HaiTang Ren.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Circular RNA SMARCA5 inhibits the proliferation, migration, and invasion of non-small cell lung cancer by miR-19b-3p/HOXA9 axis.Onco Targets Ther. 2019 Aug 30;12:7055-7065. doi: 10.2147/OTT.S216320. eCollection 2019. Onco Targets Ther. 2019. Retraction in: Onco Targets Ther. 2021 Nov 15;14:5261-5262. doi: 10.2147/OTT.S348927. PMID: 31564891 Free PMC article. Retracted.
-
A circular RNA, circSMARCA5, inhibits prostate cancer proliferative, migrative, and invasive capabilities via the miR-181b-5p/miR-17-3p-TIMP3 axis.Aging (Albany NY). 2021 Aug 13;13(15):19908-19919. doi: 10.18632/aging.203408. Epub 2021 Aug 13. Aging (Albany NY). 2021. PMID: 34390329 Free PMC article.
-
Circular RNA ITCH attenuates the progression of nasopharyngeal carcinoma by inducing PTEN upregulation via miR-214.J Gene Med. 2022 Jan;24(1):e3391. doi: 10.1002/jgm.3391. Epub 2021 Nov 11. J Gene Med. 2022. PMID: 34612550
-
CircSMARCA5 Facilitates the Progression of Prostate Cancer Through miR-432/PDCD10 Axis.Cancer Biother Radiopharm. 2021 Feb;36(1):70-83. doi: 10.1089/cbr.2019.3490. Epub 2020 May 14. Cancer Biother Radiopharm. 2021. PMID: 32407167
-
Circular RNA hsa_circ_0000317 inhibits non-small cell lung cancer progression through regulating microRNA-494-3p/phosphatase and tensin homolog deleted on chromosome 10 axis.Clinics (Sao Paulo). 2022 Jul 30;77:100086. doi: 10.1016/j.clinsp.2022.100086. eCollection 2022. Clinics (Sao Paulo). 2022. PMID: 35917658 Free PMC article.
Cited by
-
Retracted: Circular RNA SMARCA5 Modulates Epithelial-Mesenchymal Transformation, Proliferation, and Metastasis of Nasopharyngeal Carcinoma Cells via microRNA-582-3p/Phosphatase and Tensin Homolog Axis.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9876258. doi: 10.1155/2023/9876258. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125158 Free PMC article.
References
-
- Yang X. L., Wang Y., Liang S. B., et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer . 2018;18(1):p. 606. doi: 10.1186/s12885-018-4419-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

