In vivo dynamics and anti-tumor effects of EpCAM-directed CAR T-cells against brain metastases from lung cancer
- PMID: 36687005
- PMCID: PMC9851202
- DOI: 10.1080/2162402X.2022.2163781
In vivo dynamics and anti-tumor effects of EpCAM-directed CAR T-cells against brain metastases from lung cancer
Abstract
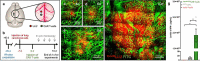
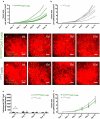
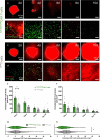
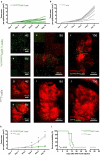
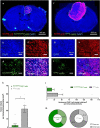
Lung cancer patients are at risk for brain metastases and often succumb to their intracranial disease. Chimeric Antigen Receptor (CAR) T-cells emerged as a powerful cell-based immunotherapy for hematological malignancies; however, it remains unclear whether CAR T-cells represent a viable therapy for brain metastases. Here, we established a syngeneic orthotopic cerebral metastasis model in mice by combining a chronic cranial window with repetitive intracerebral two-photon laser scanning-microscopy. This approach enabled in vivo-characterization of fluorescent CAR T-cells and tumor cells on a single-cell level over weeks. Intraparenchymal injection of Lewis lung carcinoma cells (expressing the tumor cell-antigen EpCAM) was performed, and EpCAM-directed CAR T-cells were injected either intravenously or into the adjacent brain parenchyma. In mice receiving EpCAM-directed CAR T-cells intravenously, we neither observed substantial CAR T-cell accumulation within the tumor nor relevant anti-tumor effects. Local CAR T-cell injection, however, resulted in intratumoral CAR T-cell accumulation compared to controls treated with T-cells lacking a CAR. This finding was accompanied by reduced tumorous growth as determined per in vivo-microscopy and immunofluorescence of excised brains and also translated into prolonged survival. However, the intratumoral number of EpCAM-directed CAR T-cells decreased during the observation period, pointing toward insufficient persistence. No CNS-specific or systemic toxicities of EpCAM-directed CAR T-cells were observed in our fully immunocompetent model. Collectively, our findings indicate that locally (but not intravenously) injected CAR T-cells may safely induce relevant anti-tumor effects in brain metastases from lung cancer. Strategies improving the intratumoral CAR T-cell persistence may further boost the therapeutic success.
Keywords: CAR T-cells; CNS tumor; adoptive immunotherapy; brain metastasis; histology; in vivo microscopy; lung cancer; survival.
© 2023 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
Tao Xu, No disclosures; Philipp Karschnia, No disclosures; Bruno L. Cadilha, No disclosures; Sertac Dede, No disclosures; Michael Lorenz, No disclosures; Niklas Seewaldt, No disclosures; Elene Nikolaishvili, No disclosures; Katharina Müller, No disclosures; Jens Blobner, No disclosures; Nico Teske, No disclosures; Julika J. Herold, No disclosures; Kai Rejeski, Kite/Gilead: Research funding and travel support, Novartis: Honoraria, BMS/CELGENE: Consultancy, Honoraria; Sigrid Langer, No disclosures; Hannah Obeck, No disclosures; Theo Lorenzini, No disclosures; Matthias Mulazzani, No disclosures; Wenlong Zhang, No disclosures; Hellen Ishikawa-Ankerhold, No disclosures; Veit R. Buchholz, No disclosures; Marion Subklewe, No disclosures; Niklas Thon, No disclosures; Andreas Straube, No disclosures; Joerg-Christian Tonn, Research grants from Novocure and Munich Surgical Imaging, and Royalties from Springer Publisher Intl; Sebastian Kobold, S.K. has received honoraria from TCR2 Inc, Novartis, BMS and GSK, S.K. is an inventor of several patents in the field of immuno-oncology, S.K. received license fees from TCR2 Inc and Carina Biotech, S.K. received research support from TCR2 Inc. and Arcus Bioscience for work unrelated to the manuscript. Louisa von Baumgarten, No disclosures.
Figures
References
-
- Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, Lilenbaum R, Wilson FH, Omay SB, Yu JB, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(5):655–663. doi: 10.1016/S1470-2045(20)30111-X. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
