Insights into the Mechanism of CRISPR/Cas9-Based Genome Editing from Molecular Dynamics Simulations
- PMID: 36687047
- PMCID: PMC9850488
- DOI: 10.1021/acsomega.2c05583
Insights into the Mechanism of CRISPR/Cas9-Based Genome Editing from Molecular Dynamics Simulations
Abstract
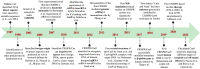
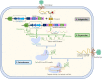
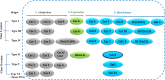
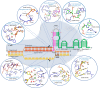
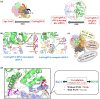
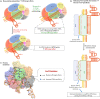
The CRISPR/Cas9 system is a popular genome-editing tool with immense therapeutic potential. It is a simple two-component system (Cas9 protein and RNA) that recognizes the DNA sequence on the basis of RNA:DNA complementarity, and the Cas9 protein catalyzes the double-stranded break in the DNA. In the past decade, near-atomic resolution structures at various stages of the CRISPR/Cas9 DNA editing pathway have been reported along with numerous experimental and computational studies. Such studies have boosted knowledge of the genome-editing mechanism. Despite such advancements, the application of CRISPR/Cas9 in therapeutics is still limited, primarily due to off-target effects. Several studies aim at engineering high-fidelity Cas9 to minimize the off-target effects. Molecular Dynamics (MD) simulations have been an excellent complement to the experimental studies for investigating the mechanism of CRISPR/Cas9 editing in terms of structure, thermodynamics, and kinetics. MD-based studies have uncovered several important molecular aspects of Cas9, such as nucleotide binding, catalytic mechanism, and off-target effects. In this Review, the contribution of MD simulation to understand the CRISPR/Cas9 mechanism has been discussed, preceded by an overview of the history, mechanism, and structural aspects of the CRISPR/Cas9 system. These studies are important for the rational design of highly specific Cas9 and will also be extremely promising for achieving more accurate genome editing in the future.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ray A.; Felice R. di; Felice R. di; Felice R. di. Molecular Simulations Have Boosted of CRISPR/Cas9: A Review. Journal of Self-Assembly and Molecular Electronics (SAME) 2019, 7 (1), 45–72. 10.13052/jsame2245-4551.7.003. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

