Recent Advances in Drug Release, Sensing, and Cellular Uptake of Ring-Opening Metathesis Polymerization (ROMP) Derived Poly(olefins)
- PMID: 36687055
- PMCID: PMC9850466
- DOI: 10.1021/acsomega.2c05563
Recent Advances in Drug Release, Sensing, and Cellular Uptake of Ring-Opening Metathesis Polymerization (ROMP) Derived Poly(olefins)
Abstract
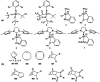
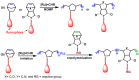
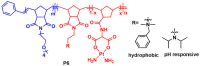
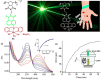
The synthesis and applications of ring-opening metathesis polymerization (ROMP) derived poly(olefins) have emerged as an exciting area of great interest in the field of biomaterials science. The major focus of this mini-review is to present recent advances in the synthesis of functional materials using ROMP-derived poly(olefins) utilized for drug release, sensing, and cellular uptake in the past seven years (2015-2022). This review reveals that materials synthesized by ROMP-derived well-defined functional poly(olefins) stand to be highly promising systems for medical as well as biological studies. Thus, this review may prove to be beneficial for the design and development of new smart and flexible-functionality ROMP-based polymeric materials for various biological applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



















Similar articles
-
Allylic Epoxides Increase the Strain Energy of Cyclic Olefin Monomers for Ring-Opening Metathesis Polymerization.Angew Chem Int Ed Engl. 2025 Jan 10;64(2):e202414872. doi: 10.1002/anie.202414872. Epub 2024 Oct 31. Angew Chem Int Ed Engl. 2025. PMID: 39320976
-
Metal-Free Ring-Opening Metathesis Polymerization: From Concept to Creation.Acc Chem Res. 2020 Oct 20;53(10):2325-2335. doi: 10.1021/acs.accounts.0c00427. Epub 2020 Sep 22. Acc Chem Res. 2020. PMID: 32960558
-
Application of olefin metathesis in the synthesis of functionalized polyhedral oligomeric silsesquioxanes (POSS) and POSS-containing polymeric materials.Beilstein J Org Chem. 2019 Feb 4;15:310-332. doi: 10.3762/bjoc.15.28. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 30800181 Free PMC article. Review.
-
Surface Chain-Transfer Ring-Opening Metathesis Polymerization.Langmuir. 2023 Nov 7;39(44):15740-15747. doi: 10.1021/acs.langmuir.3c02328. Epub 2023 Oct 30. Langmuir. 2023. PMID: 37901940
-
Grubbs' and Schrock's Catalysts, Ring Opening Metathesis Polymerization and Molecular Brushes-Synthesis, Characterization, Properties and Applications.Polymers (Basel). 2019 Feb 11;11(2):298. doi: 10.3390/polym11020298. Polymers (Basel). 2019. PMID: 30960282 Free PMC article. Review.
Cited by
-
Cu-TCPP Metal-Organic Nanosheets Embedded Thin-Film Composite Membranes for Enhanced Cyanide Detection and Removal: A Multifunctional Approach to Water Treatment and Environmental Safety.ACS Appl Mater Interfaces. 2025 Feb 12;17(6):9563-9574. doi: 10.1021/acsami.4c18944. Epub 2025 Feb 2. ACS Appl Mater Interfaces. 2025. PMID: 39893663 Free PMC article.
-
Entropy Drives the Predictive Discovery of an Optimal Cleavable Comonomer for ROMP.ACS Cent Sci. 2025 Jul 23;11(8):1408-1416. doi: 10.1021/acscentsci.5c00521. eCollection 2025 Aug 27. ACS Cent Sci. 2025. PMID: 40893968 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

