Drug-induced sarcoidosis-like reaction three months after BNT162b2 mRNA COVID-19 vaccination: A case report and review of literature
- PMID: 36687201
- PMCID: PMC9846985
- DOI: 10.12998/wjcc.v11.i1.177
Drug-induced sarcoidosis-like reaction three months after BNT162b2 mRNA COVID-19 vaccination: A case report and review of literature
Abstract
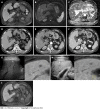
Background: A 70-year-old man with hepatitis C virus-related recurrent hepatocellular carcinoma was admitted for further diagnosis of a 1 cm iso-hyperechoic nodule in segment (S) 5.
Case summary: Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) revealed the nodule in S5 with a defect at the hepatobiliary phase, hyperintensity on diffusion weighted imaging (DWI) and hypointensity on apparent diffusion coefficient (ADC) map. Contrast-enhanced computed tomography revealed hypervascularity at the early phase, and delayed contrast-enhancement was observed at the late phase. Contrast-enhanced ultrasound (US) revealed incomplete defect at the late vascular phase. Inflammatory liver tumor, lymphoproliferative disease, intrahepatic cholangiocarcinoma (small duct type) and bile duct adenoma were suspected through the imaging studies. US guided biopsy, however, showed a noncaseating hepatic sarcoid-like epithelioid granuloma (HSEG), and histopathological analysis disclosed spindle shaped epithelioid cells harboring Langhans-type multinucleated giant cells. One month after admission, EOB-MRI signaled the disappearance of the defect at the hepatobiliary phase, of hyperintensity on DWI, of hypointensity on ADC map, and no stain at the early phase.
Conclusion: That the patient had received BNT162b2 messenger RNA (mRNA) coronavirus disease 2019 vaccination 3 mo before the occurrence of HSEG, and that its disappearance was confirmed 4 mo after mRNA vaccination suggested that the drug-induced sarcoidosis-like reaction (DISR) might be induced by the mRNA vaccination. Fortunately, rechallenge of drug-induced DISR with the third mRNA vaccination was not confirmed.
Keywords: BNT162b2 mRNA COVID-19 vaccine; Case report; Drug-induced sarcoidosis-like reaction; Ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging; Noncaseating granuloma; Th1/Th2 profile.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures


References
-
- Chopra A, Nautiyal A, Kalkanis A, Judson MA. Drug-Induced Sarcoidosis-Like Reactions. Chest. 2018;154:664–677. - PubMed
-
- Montaudié H, Pradelli J, Passeron T, Lacour JP, Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. 2017;176:1060–1063. - PubMed
-
- Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017;23:620–624. - PubMed
-
- Danlos FX, Pagès C, Baroudjian B, Vercellino L, Battistella M, Mimoun M, Jebali M, Bagot M, Tazi A, Lebbé C. Nivolumab-Induced Sarcoid-Like Granulomatous Reaction in a Patient With Advanced Melanoma. Chest. 2016;149:e133–e136. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

