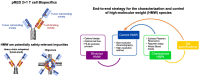
End-to-end approach for the characterization and control of product-related impurities in T cell bispecific antibody preparations
- PMID: 36687375
- PMCID: PMC9850176
- DOI: 10.1016/j.ijpx.2023.100157
End-to-end approach for the characterization and control of product-related impurities in T cell bispecific antibody preparations
Abstract
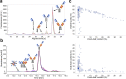
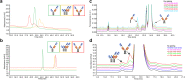
Antibody-based T cell-activating biologics are promising therapeutic medicines being developed for a number of indications, mainly in the oncology field. Among those, T cell bispecific antibodies are designed to bind one tumor-specific antigen and the T cell receptor at the same time, leading to a robust T cell response against the tumor. Although their unique format and the versatility of the CrossMab technology allows for the generation of safer molecules in an efficient manner, product-related variants cannot be completely avoided. Therefore, it is of extreme importance that both a manufacturing process that limits or depletes product-related impurities, as well as a thorough analytical characterization are in place, starting from the development of the manufacturing cell line until the assessment of potential toxicities. Here, we describe such an end-to-end approach to minimize, quantify and control impurities and -upon their functional characterization- derive specifications that allow for the release of clinical material.
Keywords: Antibody manufacturing process; CE-SDS, capillary electrophoresis‑sodium dodecyl sulfate; CRS, cytokine release syndrome; ELISA, enzyme-linked immunosorbent assay; End-to-end approach; Fc, fragment crystallizable; Functional characterization; GMP, good manufacturing process; HIC, hydrophobic interaction chromatography; HMW, high molecular weight (species); IEX, ion exchange chromatography; PBS, phosphate buffer saline; Product-related impurities control; SEC, size-exclusion chromatography; SPR, surface plasmon resonance; TAA, tumor-associated antigen; TCB, T cell bispecific; TCR, T cell receptor.
© 2023 The Authors.
Conflict of interest statement
Valeria Runza reports a relationship with Roche Diagnostics GmbH that includes: employment and equity or stocks. Laurent Lariviere reports a relationship with Roche Diagnostics GmbH that includes: employment and equity or stocks. Julia Krüger reports a relationship with Roche Diagnostics GmbH that includes: employment and equity or stocks. Tilman Schlothauer reports a relationship with Roche Diagnostics GmbH that includes: employment and equity or stocks. Martin Bader reports a relationship with Roche Diagnostics GmbH that includes: employment and equity or stocks. Katharine Bray-French reports a relationship with F. Hoffmann-La Roche AG that includes: employment and equity or stocks. Thomas von Hirschheydt reports a relationship with Roche Diagnostics GmbH that includes: employment and equity or stocks.
Figures

References
-
- Bacac M., Fauti T., Sam J., Colombetti S., Weinzierl T., Ouaret D., Bodmer W., Lehmann S., Hofer T., Hosse R.J., et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin. Cancer Res. 2016;22:3286–3297. - PubMed
-
- Bacac M., Colombetti S., Herter S., Sam J., Perro M., Chen S., Bianchi R., Richard M., Schoenle A., Nicolini V., et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin. Cancer Res. 2018;24:4785–4797. - PubMed
-
- Baeuerle P.A., Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. - PubMed
LinkOut - more resources
Full Text Sources

