TRACK-CF prospective cohort study: Understanding early cystic fibrosis lung disease
- PMID: 36687447
- PMCID: PMC9853074
- DOI: 10.3389/fmed.2022.1034290
TRACK-CF prospective cohort study: Understanding early cystic fibrosis lung disease
Abstract
Background: Lung disease as major cause for morbidity in patients with cystic fibrosis (CF) starts early in life. Its large phenotypic heterogeneity is partially explained by the genotype but other contributing factors are not well delineated. The close relationship between mucus, inflammation and infection, drives morpho-functional alterations already early in pediatric CF disease, The TRACK-CF cohort has been established to gain insight to disease onset and progression, assessed by lung function testing and imaging to capture morpho-functional changes and to associate these with risk and protective factors, which contribute to the variation of the CF lung disease progression.
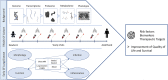
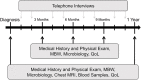
Methods and design: TRACK-CF is a prospective, longitudinal, observational cohort study following patients with CF from newborn screening or clinical diagnosis throughout childhood. The study protocol includes monthly telephone interviews, quarterly visits with microbiological sampling and multiple-breath washout and as well as a yearly chest magnetic resonance imaging. A parallel biobank has been set up to enable the translation from the deeply phenotyped cohort to the validation of relevant biomarkers. The main goal is to determine influencing factors by the combined analysis of clinical information and biomaterials. Primary endpoints are the lung clearance index by multiple breath washout and semi-quantitative magnetic resonance imaging scores. The frequency of pulmonary exacerbations, infection with pro-inflammatory pathogens and anthropometric data are defined as secondary endpoints.
Discussion: This extensive cohort includes children after diagnosis with comprehensive monitoring throughout childhood. The unique composition and the use of validated, sensitive methods with the attached biobank bears the potential to decisively advance the understanding of early CF lung disease.
Ethics and trial registration: The study protocol was approved by the Ethics Committees of the University of Heidelberg (approval S-211/2011) and each participating site and is registered at clinicaltrials.gov (NCT02270476).
Keywords: biomarkers in cystic fibrosis; cystic fibrosis; early lung disease; magnetic resonance imaging (MRI); multiple-breath washout (MBW); non-invasive monitoring; risk factors in cystic fibrosis.
Copyright © 2023 Steinke, Sommerburg, Graeber, Joachim, Labitzke, Nissen, Ricklefs, Rudolf, Kopp, Dittrich, Mall and Stahl.
Conflict of interest statement
OS reports grants by the German Center for Lung Research (DZL) and Vertex Pharmaceuticals with payments to the institution. SG reports grants by the Mukoviszidose e.V., Vertex Pharmaceuticals and the German Research Foundation (DFG) with payments to the institution and received personal fees from Chiesi GmbH and Vertex Pharmaceuticals. MK reports grants by the German Center for Lung Research (DZL) and received personal fees from Sanofi Aventis GmbH, Chiesi GmbH, Allergopharma GmbH, Infectopharm GmbH, Vertex Pharma GmbH, Leti GmbH and Nutricia GmbH. A-MD reports grants by the German Center for Lung Research (DZL) and Vertex Pharmaceuticals with payments to the institution and received personal fees from Vertex Pharmaceuticals. MS reports grants by the Mukoviszidose e.V., the German Center for Lung Research (DZL), the Christiane Herzog Foundation and Vertex Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

