Betulinic acid analogs inhibit N- and T-type voltage-gated calcium channels to attenuate nerve-injury associated neuropathic and formalin models of pain
- PMID: 36687466
- PMCID: PMC9853350
- DOI: 10.1016/j.ynpai.2023.100116
Betulinic acid analogs inhibit N- and T-type voltage-gated calcium channels to attenuate nerve-injury associated neuropathic and formalin models of pain
Abstract
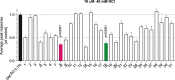
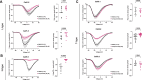
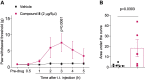
Over the past three decades, there has been a significant growth in the use of natural products, with approximately 80% of individuals using them for some aspect of primary healthcare. Our laboratories have identified and studied natural compounds with analgesic effects from dry land plants or their associated fungus during the past ten years. Here, we isolated and characterized thirteen betulin analogs and fifteen betulinic acid analogs for their capacity to prevent calcium influx brought on by depolarization in sensory neurons. The in vitro inhibition of voltage-gated calcium channels by the top drugs was then assessed using whole cell patch clamp electrophysiology. In vivo experiments, conducted at two sites, evaluated the best compound in acute and tonic, neuropathic, inflammatory, post-operative and visceral models of pain. We found that the betulinic acid analog 8 inhibited calcium influx in rat dorsal root ganglion neurons by inhibiting N- (CaV2.2) and T- (CaV3) type voltage-gated calcium channels. Moreover, intrathecal delivery of analog 8 had analgesic activity in both spared nerve injury model of neuropathic pain and acute and tonic pain induced by formalin. The results presented herein highlight the potential antinociceptive properties of betulinic acid analog 8 and set the stage for the development of novel non-opioid pain therapeutics based on the triterpenoid scaffold of betulinic acid.
Keywords: Analgesic; BA, Betulinic acid; Betulin analogs; Betulinic acid analogs; CaV2.2, N-type voltage-gated calcium channel; CaV3, T-type voltage-gated calcium channel; DRG, dorsal root ganglia; Formalin model; HVA, high voltage-gated; LVA, low voltage-gated; Pain; SNI, spared nerve injury; Spared nerve injury mode; VGCCs, Voltage-gated calcium channels; Voltage-gated calcium channels.
© 2023 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: R.K. is the founder of Regulonix LLC, a company developing nonopioid drugs for chronic pain. In addition, R.K., has patents US10287334 (non-narcotic CRMP2 peptides targeting sodium channels for chronic pain) and US10441586 (SUMOylation inhibitors and uses thereof) issued to Regulonix LLC. The other authors declare no conflicts.
Figures




Similar articles
-
Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels.Pain. 2019 Jan;160(1):117-135. doi: 10.1097/j.pain.0000000000001385. Pain. 2019. PMID: 30169422 Free PMC article.
-
Reversal of Peripheral Neuropathic Pain by the Small-Molecule Natural Product Physalin F via Block of CaV2.3 (R-Type) and CaV2.2 (N-Type) Voltage-Gated Calcium Channels.ACS Chem Neurosci. 2019 Jun 19;10(6):2939-2955. doi: 10.1021/acschemneuro.9b00166. Epub 2019 Apr 18. ACS Chem Neurosci. 2019. PMID: 30946560
-
The natural product argentatin C attenuates postoperative pain via inhibition of voltage-gated sodium and T-type voltage-gated calcium channels.Br J Pharmacol. 2023 May;180(9):1267-1285. doi: 10.1111/bph.15974. Epub 2023 Jan 15. Br J Pharmacol. 2023. PMID: 36245395
-
Involvement of Voltage-Gated Calcium Channels in Inflammation and Inflammatory Pain.Biol Pharm Bull. 2018;41(8):1127-1134. doi: 10.1248/bpb.b18-00054. Biol Pharm Bull. 2018. PMID: 30068860 Review.
-
Role of Cav2.3 (R-type) Calcium Channel in Pain and Analgesia: A Scoping Review.Curr Neuropharmacol. 2024;22(11):1909-1922. doi: 10.2174/1570159X21666230811102700. Curr Neuropharmacol. 2024. PMID: 37581322 Free PMC article.
Cited by
-
Inhibition of N-type calcium channels by phenoxyaniline and sulfonamide analogues.RSC Med Chem. 2024 Jan 31;15(3):916-936. doi: 10.1039/d3md00714f. eCollection 2024 Mar 20. RSC Med Chem. 2024. PMID: 38516585 Free PMC article.
-
Phytochemical Profiling and Antioxidant Activities of the Most Favored Ready-to-Use Thai Curries, Pad-Ka-Proa (Spicy Basil Leaves) and Massaman.Foods. 2024 Feb 14;13(4):582. doi: 10.3390/foods13040582. Foods. 2024. PMID: 38397559 Free PMC article.
-
Regulation of pain neurotransmitters and chondrocytes metabolism mediated by voltage-gated ion channels: A narrative review.Heliyon. 2023 Jul 5;9(7):e17989. doi: 10.1016/j.heliyon.2023.e17989. eCollection 2023 Jul. Heliyon. 2023. PMID: 37501995 Free PMC article. Review.
-
Ethanolic Extracts of Cissus quadrangularis Linn. (Vitaceae) Attenuate Vincristine-Induced Peripheral Neuropathy in Rats: An Evidence of the Antioxidant, Calcium Inhibitory, and Neuromodulatory Properties.Adv Pharmacol Pharm Sci. 2024 Dec 12;2024:8822369. doi: 10.1155/adpp/8822369. eCollection 2024. Adv Pharmacol Pharm Sci. 2024. PMID: 39703364 Free PMC article.
-
Exploring the Analgesic Potential of L-Lysine: Molecular Mechanisms, Preclinical Evidence, and Implications for Pharmaceutical Pain Therapy.Pharmaceutics. 2025 May 19;17(5):666. doi: 10.3390/pharmaceutics17050666. Pharmaceutics. 2025. PMID: 40430956 Free PMC article. Review.
References
-
- Alles S.R.A., Smith P.A. 2017. The anti-allodynic gabapentinoids: myths, paradoxes, and acute effects. Neuroscientist. 23(1):40-55. Epub 20160708. doi: 10.1177/1073858416628793. PubMed PMID: 27118808. - PubMed
-
- Bauer C.S., Nieto-Rostro M., Rahman W., Tran-Van-Minh A., Ferron L., Douglas L., et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29(13):4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. PubMed PMID: 19339603; PubMed Central PMCID: PMC6665374. - DOI - PMC - PubMed
-
- Bellampalli S.S., Ji Y., Moutal A., Cai S., Wijeratne E.M.K., Gandini M.A., et al. Betulinic acid, derived from the desert lavender Hyptis emoryi, attenuates paclitaxel-, HIV-, and nerve injury-associated peripheral sensory neuropathy via block of N- and T-type calcium channels. Pain. 2019;160(1):117–135. doi: 10.1097/j.pain.0000000000001385. PubMed PMID: 30169422; PubMed Central PMCID: PMC6309937. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

